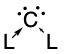|
Carbones
Carbones are a class of molecules containing a carbon atom in the 1D excited state with a formal oxidation state of zero where all four Valence electron, valence electrons exist as unbonded lone pairs. These carbon-based compounds are of the formula CL2 where L is a strongly Sigma bond, σ-donating ligand, typically a phosphine (carbodiphosphoranes) or a Carbene, N-heterocyclic carbene/NHC (carbodicarbenes), that stabilises the central carbon atom through Coordinate covalent bond, donor-acceptor bonds. Carbones possess high-energy Molecular orbital, orbitals with both σ- and Pi bond, π-symmetry, making them strong Lewis acids and bases, Lewis bases and strong Pi backbonding, π-backdonor substituents. Carbones possess high Proton affinity, proton affinities and are strong Nucleophile, nucleophiles which allows them to function as ligands in a variety of Main-group element, main group and transition metal complexes. Carbone-coordinated elements also exhibit a variety of di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Coordinate Covalent Bond
In coordination chemistry, a coordinate covalent bond, also known as a dative bond, dipolar bond, or coordinate bond is a kind of two-center, two-electron covalent bond in which the two electrons derive from the same atom. The bonding of metal ions to ligands involves this kind of interaction. This type of interaction is central to Lewis acid–base theory. Coordinate bonds are commonly found in coordination compounds. __TOC__ Examples Coordinate covalent bonding is ubiquitous. In all metal aquo-complexes (H2O)''n'''m''+, the bonding between water and the metal cation is described as a coordinate covalent bond. Metal-ligand interactions in most organometallic compounds and most coordination compounds are described similarly. The term ''dipolar bond'' is used in organic chemistry for compounds such as amine oxides for which the electronic structure can be described in terms of the basic amine donating two electrons to an oxygen atom. : → O The arrow → indicate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Proton Affinity
The proton affinity (PA, ''E''pa) of an anion or of a neutral atom or molecule is the negative of the enthalpy change in the reaction between the chemical species concerned and a proton in the gas phase: ::: A- + H+ -> HA ::: B + H+ -> BH+ These reactions are always exothermic in the gas phase, i.e. energy is released ( enthalpy is negative) when the reaction advances in the direction shown above, while the proton affinity is positive. This is the same sign convention used for electron affinity. The property related to the proton affinity is the gas-phase basicity, which is the negative of the Gibbs energy for above reactions, i.e. the gas-phase basicity includes entropic terms in contrast to the proton affinity. Acid/base chemistry The higher the proton affinity, the stronger the base and the weaker the conjugate acid ''in the gas phase''. The (reportedly) strongest known base is the ortho-diethynylbenzene dianion (''E''pa = 1843 kJ/mol), followed by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hexaphenylcarbodiphosphorane
Hexaphenylcarbodiphosphorane is the organophosphorus compound with the formula C(PPh3)2 (where Ph = C6H5). It is a yellow, moisture-sensitive solid. The compound is classified as an ylide and as such carries significant negative charge on carbon. It is isoelectronic with bis(triphenylphosphine)iminium. The P-C-P angle is 131°. The compound has attracted attention as an unusual ligand in organometallic chemistry. The pure compound has two crystalline phases: a metastable monoclinic C2 phase that is triboluminescent, and an orthorhombic P222 form that is not. Both polymorphs are photoluminescent, with respective peak wavelengths at 540 and 575 nm. Preparation The compound was originally prepared by deprotonation of the phosphonium salt In chemistry, the term phosphonium (more obscurely: phosphinium) describes polyatomic cations with the chemical formula (where R is a hydrogen or an alkyl, aryl, organyl or halogen group). These cations have tetrahedral structures. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Zwitterion
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively and negatively charged functional groups. : (1,2- dipolar compounds, such as ylides, are sometimes excluded from the definition.) Some zwitterions, such as amino acid zwitterions, are in chemical equilibrium with an uncharged "parent" molecule. Betaines are zwitterions that cannot isomerize to an all-neutral form, such as when the positive charge is located on a quaternary ammonium group. Similarly, a molecule containing a phosphonium group and a carboxylate group cannot isomerize. Amino acids Tautomerism of amino acids follows this stoichiometry: : The ratio of the concentrations of the two species in solution is independent of pH. It has been suggested, on the basis of theoretical analysis, that the zwitterion is stabilized in aqueous solution by hydrogen bonding with solvent water molecules. Analysis of neutron diffraction data for g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared artificially, the two most common allotropes being white phosphorus and red phosphorus. With as its only stable isotope, phosphorus has an occurrence in Earth's crust of about 0.1%, generally as phosphate rock. A member of the pnictogen family, phosphorus readily forms a wide variety of organic compound, organic and inorganic compound, inorganic compounds, with as its main oxidation states +5, +3 and −3. The isolation of white phosphorus in 1669 by Hennig Brand marked the scientific community's first discovery since Antiquity of an element. The name phosphorus is a reference to the Phosphorus (morning star), god of the Morning star in Greek mythology, inspired by the faint glow of white phosphorus when exposed to oxygen. This property is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Resonance (chemistry)
In chemistry, resonance, also called mesomerism, is a way of describing Chemical bond, bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ''canonical structures'') into a resonance hybrid (or ''hybrid structure'') in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure. The resonance hybrid is the accurate structure for a molecule or ion; it is an average of the theoretical (or hypothetical) contributing structures. Overview Under the framework of valence bond theory, resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure. For many chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charges, and connected by bonds of positive integer order, is suffi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chemical Synthesis
Chemical synthesis (chemical combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses, the process is reproducible and reliable. A chemical synthesis involves one or more compounds (known as '' reagents'' or ''reactants'') that will experience a transformation under certain conditions. Various reaction types can be applied to formulate a desired product. This requires mixing the compounds in a reaction vessel, such as a chemical reactor or a simple round-bottom flask. Many reactions require some form of processing (" work-up") or purification procedure to isolate the final product. The amount produced by chemical synthesis is known as the '' reaction yield''. Typically, yields are expressed as a mass in grams (in a laboratory setting) or as a percentage of the total theoretical quantity that could be produced based ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chemical Reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, energy change as new products are generated. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Catalysis
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. The rate increase occurs because the catalyst allows the reaction to occur by an alternative mechanism which may be much faster than the noncatalyzed mechanism. However the noncatalyzed mechanism does remain possible, so that the total rate (catalyzed plus noncatalyzed) can only increase in the presence of the catalyst and never decrease. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |