|
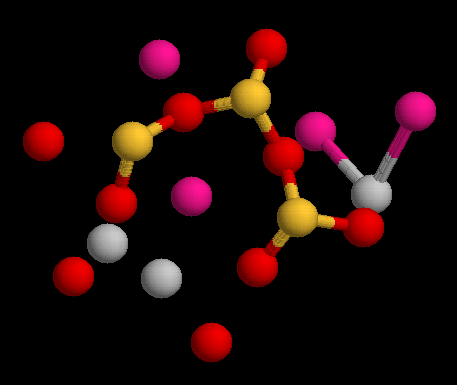
Calcium Silicate Hydrate
Calcium silicate hydrates (CSH or C-S-H) are the main products of the hydration of Portland cement and are primarily responsible for the strength of cement-based materials. They are the main binding phase (the "glue") in most concrete. Only well defined and rare natural crystalline minerals can be abbreviated as CSH while extremely variable and poorly ordered phases without well defined stoichiometry, as it is commonly observed in hardened cement paste (HCP), are denoted C-S-H. Preparation When water is added to cement, each of the compounds undergoes hydration and contributes to the final state of the concrete. Only calcium silicates contribute to the strength. Tricalcium silicate is responsible for most of the early strength (first 7 days). Dicalcium silicate, which reacts more slowly, only contributes to late strength. Calcium silicate hydrate (also shown as C-S-H) is a result of the reaction between the silicate phases of Portland cement and water. This reaction typically is ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Portland Cement
Portland cement is the most common type of cement in general use around the world as a basic ingredient of concrete, mortar (masonry), mortar, stucco, and non-specialty grout. It was developed from other types of hydraulic lime in England in the early 19th century by Joseph Aspdin, and is usually made from limestone. It is a fine powder (substance), powder, produced by heating limestone and clay minerals in a kiln to form Clinker (cement), clinker, and then #Cement grinding, grinding the clinker with the addition of several percent (often around 5%) gypsum. Several types of portland cement are available. The most common, historically called ordinary portland cement (OPC), is grey, but white portland cement is also available. The cement was so named by Joseph Aspdin, who obtained a patent for it in 1824, because, once hardened, it resembled the fine, pale limestone known as Portland stone, quarried from the windswept cliffs of the Isle of Portland in Dorset. Portland stone was p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
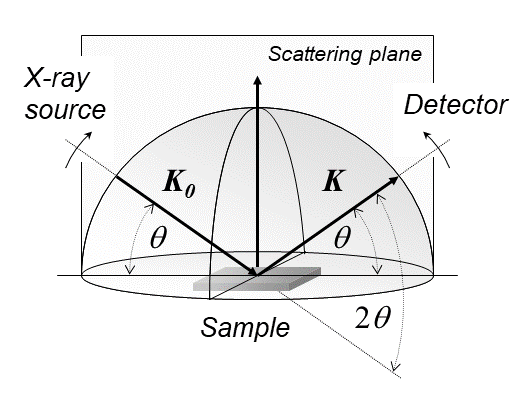
X-ray Diffraction
X-ray diffraction is a generic term for phenomena associated with changes in the direction of X-ray beams due to interactions with the electrons around atoms. It occurs due to elastic scattering, when there is no change in the energy of the waves. The resulting map of the directions of the X-rays far from the sample is called a diffraction pattern. It is different from X-ray crystallography which exploits X-ray diffraction to determine the arrangement of atoms in materials, and also has other components such as ways to map from experimental diffraction measurements to the positions of atoms. This article provides an overview of X-ray diffraction, starting with the early #History, history of x-rays and the discovery that they have the right spacings to be diffracted by crystals. In many cases these diffraction patterns can be #Introduction to x-ray diffraction theory, Interpreted using a single scattering or kinematical theory with conservation of energy (#Ewald's sphere, wave vecto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Inorganic Compounds
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. All allotropes (structurally different pure forms of an element) and some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, graphene, etc.), carbon monoxide , carbon dioxide , carbides, and salts of inorganic anions such as carbonates, cyanides, cyanates, thiocyanates, isothiocyanates, etc. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it cannot occur within living things. History Friedrich Wöhler's conver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hydrates
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood. Chemical nature Inorganic chemistry Hydrates are not inorganic salts "containing water molecules combined in a definite ratio as an integral part of the crystal" that are either bound to a metal center or that have crystallized with the metal complex. Such hydrates are also said to contain ''water of crystallization'' or ''water of hydration''. If the water is heavy water in which the constituent hydrogen is the isotope deuterium, then the term ''deuterate'' may be used in place of ''hydrate''. A colorful example is cobalt(II) chloride, which turns from blue to red upon hydration, and can therefore be used as a water indicator. The notation "''hydrated compound''⋅''n''", where ''n'' is the number of water molecules per formu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Concrete
Concrete is a composite material composed of aggregate bound together with a fluid cement that cures to a solid over time. It is the second-most-used substance (after water), the most–widely used building material, and the most-manufactured material in the world. When aggregate is mixed with dry Portland cement and water, the mixture forms a fluid slurry that can be poured and molded into shape. The cement reacts with the water through a process called hydration, which hardens it after several hours to form a solid matrix that binds the materials together into a durable stone-like material with various uses. This time allows concrete to not only be cast in forms, but also to have a variety of tooled processes performed. The hydration process is exothermic, which means that ambient temperature plays a significant role in how long it takes concrete to set. Often, additives (such as pozzolans or superplasticizers) are included in the mixture to improve the physical prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Cement
A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel ( aggregate) together. Cement mixed with fine aggregate produces mortar for masonry, or with sand and gravel, produces concrete. Concrete is the most widely used material in existence and is behind only water as the planet's most-consumed resource. Cements used in construction are usually inorganic, often lime- or calcium silicate-based, and are either hydraulic or less commonly non-hydraulic, depending on the ability of the cement to set in the presence of water (see hydraulic and non-hydraulic lime plaster). Hydraulic cements (e.g., Portland cement) set and become adhesive through a chemical reaction between the dry ingredients and water. The chemical reaction results in mineral hydrates that are not very water-soluble. This allows setting in wet conditions or u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Calcium Compounds
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossils of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name comes from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharmaceuticals for calc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nuclear Magnetic Resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. High-resolution nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crysta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Dynamic Nuclear Polarisation
Dynamic nuclear polarization (DNP) is one of several Hyperpolarization (physics), hyperpolarization methods developed to enhance the sensitivity of nuclear magnetic resonance (NMR) spectroscopy. While an essential analytical tool with applications in several fields, NMR’s low sensitivity poses major limitations to analyzing samples with low concentrations and limited masses and volumes.Abhyankar, N., & Szalai, V. (2021). Challenges and advances in the application of dynamic nuclear polarization to liquid-state NMR spectroscopy. ''The Journal of Physical Chemistry B'', ''125''(20), 5171–5190. https://doi.org/10.1021/acs.jpcb.0c10937 This low sensitivity is due to the relatively low nuclear gyromagnetic ratios (''γn'') of NMR active nuclei (1H, 13C, 15N, etc.) as well as the low natural abundance of certain nuclei.Plainchont, B., Berruyer, P., Dumez, J.-N., Jannin, S., & Giraudeau, P. (2018). Dynamic nuclear polarization opens new perspectives for NMR spectroscopy in Analytica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Tobermorite
Tobermorite is a calcium silicate hydrate mineral with chemical formula: Ca5Si6O16(OH)2·4H2O or Ca5Si6(O,OH)18·5H2O. Two structural varieties are distinguished: tobermorite-11 Å and tobermorite-14 Å. Tobermorite occurs in hydrated cement paste and can be found in nature as an alteration mineral in metamorphosed limestone and in skarn. It has been reported to occur in the Maqarin Area of north Jordan and in the Crestmore Quarry near Crestmore Heights, Riverside County, California. Tobermorite was first described in 1880 for an occurrence in Scotland, on the Isle of Mull, around the locality of Tobermory. Use in Roman concrete Aluminum-substituted tobermorite is understood to be a key ingredient responsible for the longevity of ancient undersea Roman concrete. The volcanic ash that Romans used for construction of sea walls contained phillipsite, and an interaction with sea water caused the crystalline structures in the concrete to expand and strengthen, making that mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ammonium Nitrate
Ammonium nitrate is a chemical compound with the formula . It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, but does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer. Its other major use is as a component of explosive mixtures used in mining, quarrying, and civil construction. It is the major constituent of ANFO, an industrial explosive which accounts for 80% of explosives used in North America; similar formulations have been used in improvised explosive devices. Many countries are phasing out its use in consumer applications due to concerns over its potential for misuse.Ammonium nitrate sold by ton as U.S. regulati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Concrete Strength
Concrete is a composite material composed of aggregate bound together with a fluid cement that cures to a solid over time. It is the second-most-used substance (after water), the most–widely used building material, and the most-manufactured material in the world. When aggregate is mixed with dry Portland cement and water, the mixture forms a fluid slurry that can be poured and molded into shape. The cement reacts with the water through a process called hydration, which hardens it after several hours to form a solid matrix that binds the materials together into a durable stone-like material with various uses. This time allows concrete to not only be cast in forms, but also to have a variety of tooled processes performed. The hydration process is exothermic, which means that ambient temperature plays a significant role in how long it takes concrete to set. Often, additives (such as pozzolans or superplasticizers) are included in the mixture to improve the physical properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |