|
Benzoin Condensation
In organic chemistry, the benzoin addition is an addition reaction involving two aldehydes (). The reaction generally occurs between aromatic compound, aromatic aldehydes or glyoxals (), and results in formation of an acyloin (). In the classic example, benzaldehyde is converted to Benzoin (organic compound), benzoin (). The benzoin condensation was first reported in 1832 by Justus von Liebig and Friedrich Wöhler during their research on almond, bitter almond oil. The catalytic version of the reaction involving cyanide was developed by Nikolay Zinin in the late 1830s. Reaction mechanism The reaction is catalysis, catalyzed by nucleophile, nucleophiles such as a cyanide or an persistent carbene, N-heterocyclic carbene (usually Thiazolium salt, thiazolium salts). The reaction mechanism was proposed in 1903 by Arthur Lapworth, A. J. Lapworth. In the first step in this reaction, the cyanide anion (as sodium cyanide) reacts with the aldehyde in a nucleophilic addition. Rearrangemen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
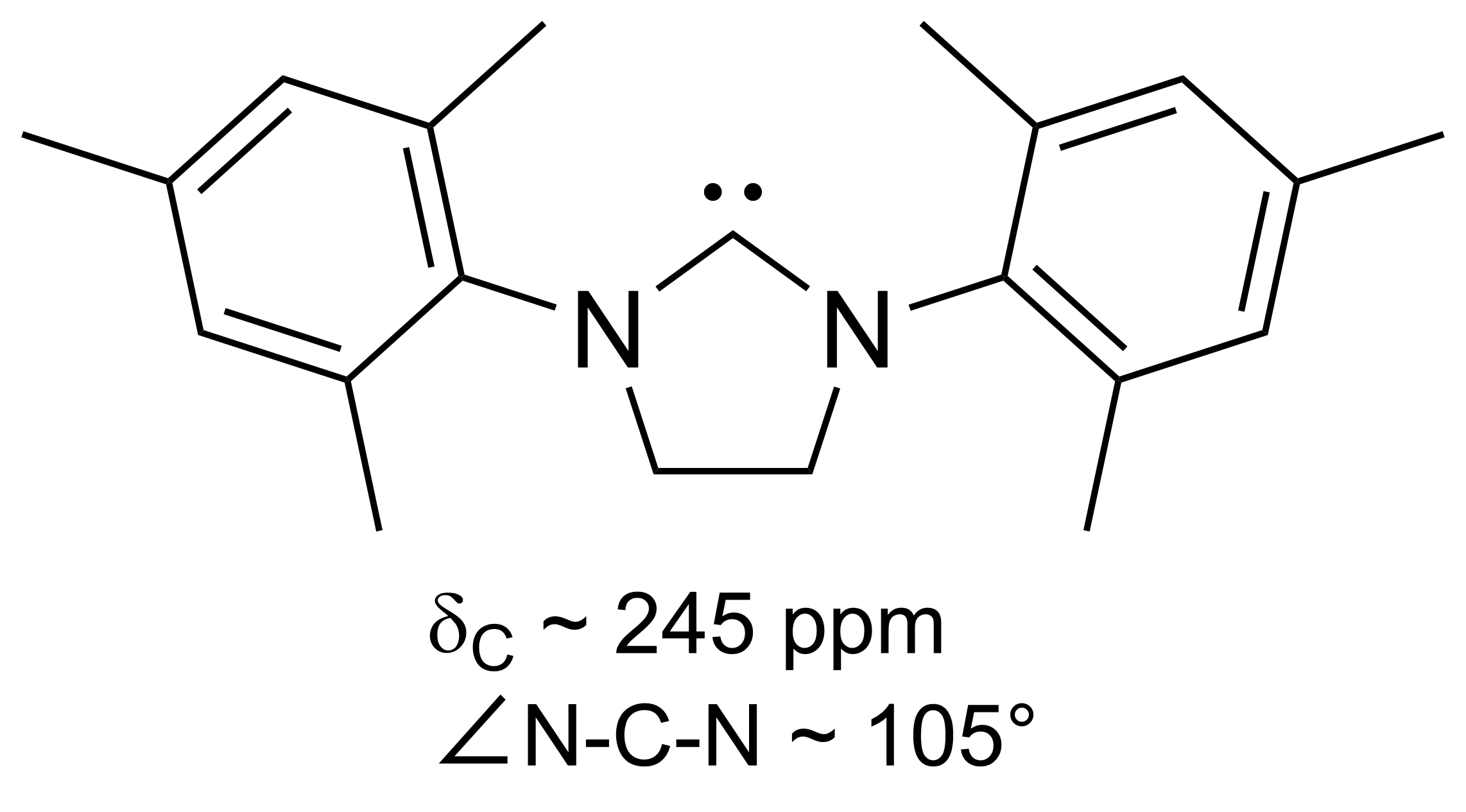
Persistent Carbene
A persistent carbene (also known as stable carbene) is an organic molecule whose natural resonance structure has a carbon atom with octet rule, incomplete octet (a carbene), but does not exhibit the tremendous instability typically associated with such moieties. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), in which nitrogen atoms flank the formal carbene. Modern theoretical analysis suggests that the term "persistent carbene" is in fact a misnomer. Persistent carbenes do not in fact have a carbene electronic structure in their ground state, but instead an ylide stabilized by Aromaticity, aromatic resonance or steric shielding. Excitation to a carbene structure then accounts for the carbene-like dimerization that some persistent carbenes undergo over the course of days. Persistent carbenes in general, and Arduengo carbenes in particular, are popular ligands in organometallic chemistry. Histor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aliphatic Compound
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated (in which all the C-C bonds are single, requiring the structure to be completed, or 'saturated', by hydrogen) like hexane, or unsaturated, like hexene and hexyne. Open-chain compounds, whether straight or branched, and which contain no rings of any type, are always aliphatic. Cyclic compounds can be aliphatic if they are not aromatic. Structure Aliphatics compounds can be saturated, joined by single bonds ( alkanes), or unsaturated, with double bonds ( alkenes) or triple bonds ( alkynes). If other elements ( heteroatoms) are bound to the carbon chain, the most common being oxygen, nitrogen, sulfur, and chlorine, it is no longer a hydrocarbon, and therefore no longer an aliphatic compound. However, such compounds may still be referred to as al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Para-dimethylaminobenzaldehyde
''para''-Dimethylaminobenzaldehyde is an organic compound containing amine and aldehyde moieties which is used in Ehrlich's reagent and Kovac's reagent to test for indoles. The carbonyl group typically reacts with the electron rich 2-position of the indole but may also react at the C-3 or N-1 positions. It may also be used for determination of hydrazine. Ehrlich's reagent ''para''-Dimethylaminobenzaldehyde is the main ingredient in Ehrlich's reagent. It acts as a strong electrophile which reacts with the electron-rich α-carbon (2-position) of indole rings to form a blue-colored adduct. It can be used to detect the presence of indole alkaloids. Not all indole alkaloids give a colored adduct as result of steric hindrance which does not allow the reaction to proceed. Ehrlich's reagent is also used as a stain in thin layer chromatography and as a reagent to detect urobilinogen in fresh, cool urine. If a urine sample is left to oxidize in air to form urobilin the reagent will ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoin Mechanism
Benzoin may refer to: *Benzoin (organic compound), an organic compound with the formula PhCH(OH)C(O)Ph *Benzoin (resin), a balsamic resin obtained from the bark of several species of trees in the genus Styrax *Benzoin aldolase, an enzyme that catalyzes the chemical reaction benzoin to benzaldehyde *Benzoin condensation, a reaction between two aromatic aldehydes * Benzoin odoriferum or Lindera benzoin, a shrub in the laurel family * Benzoin tree, the common name of Styrax, a genus of shrubs or trees in the family Styracaceae *Tincture of benzoin, a pungent solution of benzoin resin in ethanol See also * C14H12O2, molecular formula of benzoin *Benzene (C6H6), organic chemical compound of hydrocarbon class *Benzoic acid Benzoic acid () is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which ... (or C6H5 [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Stability
In chemistry, chemical stability is the thermodynamic stability of a chemical system, in particular a chemical compound or a polymer. Colloquially, it may instead refer to kinetic persistence, the shelf-life of a metastable substance or system; that is, the timescale over which it begins to degrade. Thermodynamic stability occurs when a system is in its lowest energy state, or in chemical equilibrium with its environment. This may be a dynamic equilibrium in which individual atoms or molecules change form, but their overall number in a particular form is conserved. This type of chemical thermodynamic equilibrium will persist indefinitely unless the system is changed. Chemical systems might undergo changes in the phase of matter or a set of chemical reactions. State A is said to be more thermodynamically stable than state B if the Gibbs free energy of the change from A to B is positive. Versus reactivity Thermodynamic stability applies to a particular system. The reactivity of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reversible Reaction
A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur simultaneously. : \mathit aA + \mathit bB \mathit cC + \mathit dD A and B can react to form C and D or, in the reverse reaction, C and D can react to form A and B. This is distinct from a reversible process in thermodynamics. Weak acids and bases undergo reversible reactions. For example, carbonic acid: : H2CO3 (l) + H2O(l) ⇌ HCO3−(aq) + H3O+(aq). The concentrations of reactants and products in an equilibrium mixture are determined by the analytical concentrations of the reagents (A and B or C and D) and the equilibrium constant, ''K''. The magnitude of the equilibrium constant depends on the Gibbs free energy change for the reaction. So, when the free energy change is large (more than about 30 kJ mol−1), the equilibrium constant is large (log K > 3) and the concentrations of the reactants at equilibrium are very small. Such a reaction i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Ion
A hydrogen ion is created when a hydrogen atom loses or gains an electron. A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particle-free space. Due to its extremely high charge density of approximately 2×1010 times that of a sodium ion, the bare hydrogen ion cannot exist freely in solution as it readily hydrates, i.e., bonds quickly. The hydrogen ion is recommended by International Union of Pure and Applied Chemistry, IUPAC as a general term for all ions of hydrogen and its isotopes. Depending on the electric charge, charge of the ion, two different classes can be distinguished: positively charged ions (hydrons) and negatively charged (hydride) ions. Cation (positively charged) A hydrogen atom is made up of a nucleus with charge +1, and a single electron. Therefore, the only positively charged ion possible has charge +1. It is noted H+. Depending on the isoto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes, ketones and carboxylic acid), as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, such that carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides, chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, Hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Umpolung
In organic chemistry, umpolung () or polarity inversion is the chemical modification of a functional group with the aim of the reversal of Chemical polarity, polarity of that group. This modification allows secondary reactions of this functional group that would otherwise not be possible. The concept was introduced by D. Seebach (hence the German word for reversed polarity) and E.J. Corey. Polarity analysis during retrosynthetic analysis tells a chemist when umpolung tactics are required to synthesize a target molecule. Introduction The vast majority of important organic molecules contain heteroatoms, which polarize carbon skeletons by virtue of their electronegativity. Therefore, in standard organic reactions, the majority of new bonds are formed between atoms of opposite polarity. This can be considered to be the "normal" mode of reactivity. One consequence of this natural polarization of molecules is that 1,3- and 1,5- heteroatom substituted carbon skeletons are extremely eas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Addition
In organic chemistry, a nucleophilic addition (AN) reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions differ from electrophilic additions in that the former reactions involve the group to which atoms are added accepting electron pairs, whereas the latter reactions involve the group donating electron pairs. Addition to carbon–heteroatom double bonds Nucleophilic addition reactions of nucleophiles with electrophilic double or triple bond (π bonds) create a new carbon center with two additional single, or σ, bonds.March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. Addition of a nucleophile to carbon–heteroatom double or triple bonds such as >C=O or -C≡N show great variety. These types of bonds are polar (have a large difference in electronegativit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Cyanide
Sodium cyanide is a compound with the formula Na C N and the structure . It is a white, water-soluble solid. Cyanide has a high affinity for metals, which leads to the high toxicity of this salt. Its main application, in gold mining, also exploits its high reactivity toward metals. It is a moderately strong base. Production and chemical properties Sodium cyanide is produced by treating hydrogen cyanide with sodium hydroxide: : Worldwide production was estimated at 500,000 tons in the year 2006. Formerly it was prepared by the Castner process involving the reaction of sodium amide with carbon at elevated temperatures. : The structure of solid NaCN is related to that of sodium chloride. The anions and cations are each six-coordinate. Potassium cyanide (KCN) adopts a similar structure. When treated with acid, it forms the toxic gas hydrogen cyanide: : Because the salt is derived from a weak acid, sodium cyanide readily reverts to HCN by hydrolysis; the moist solid emits smal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |