|
Basic Aromatic Ring
Basic aromatic rings are aromatic rings in which the lone pair of electrons of a ring-nitrogen atom is not part of the aromatic system and extends in the plane of the ring. This lone pair is responsible for the basicity of these nitrogenous bases, similar to the nitrogen atom in amines. In these compounds the nitrogen atom is not connected to a hydrogen atom. Basic aromatic compounds get protonated and form aromatic cations (e.g. pyridinium) under acidic conditions. Typical examples of basic aromatic rings are pyridine or quinoline. Several rings contain basic as well as non-basic nitrogen atoms, e.g. imidazole and purine. In non-basic aromatic rings the lone pair of electrons of the nitrogen atom is delocalized and contributes to the aromatic pi electron system. In these compounds the nitrogen atom is connected to a hydrogen atom. Examples of non-basic nitrogen-containing aromatic rings are pyrrole and indole. Pyrrole contains a lone pair that is part of the pi-conjugated s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridine Structure
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide. Properties Physical properties The molecular electric dipole moment is 2.2 debyes. Pyridine is diamagnetism, diamagnetic and has a Magnetic susceptibility, diamagnetic susceptibility of −48.7 × 10−6 cm3·mol−1. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
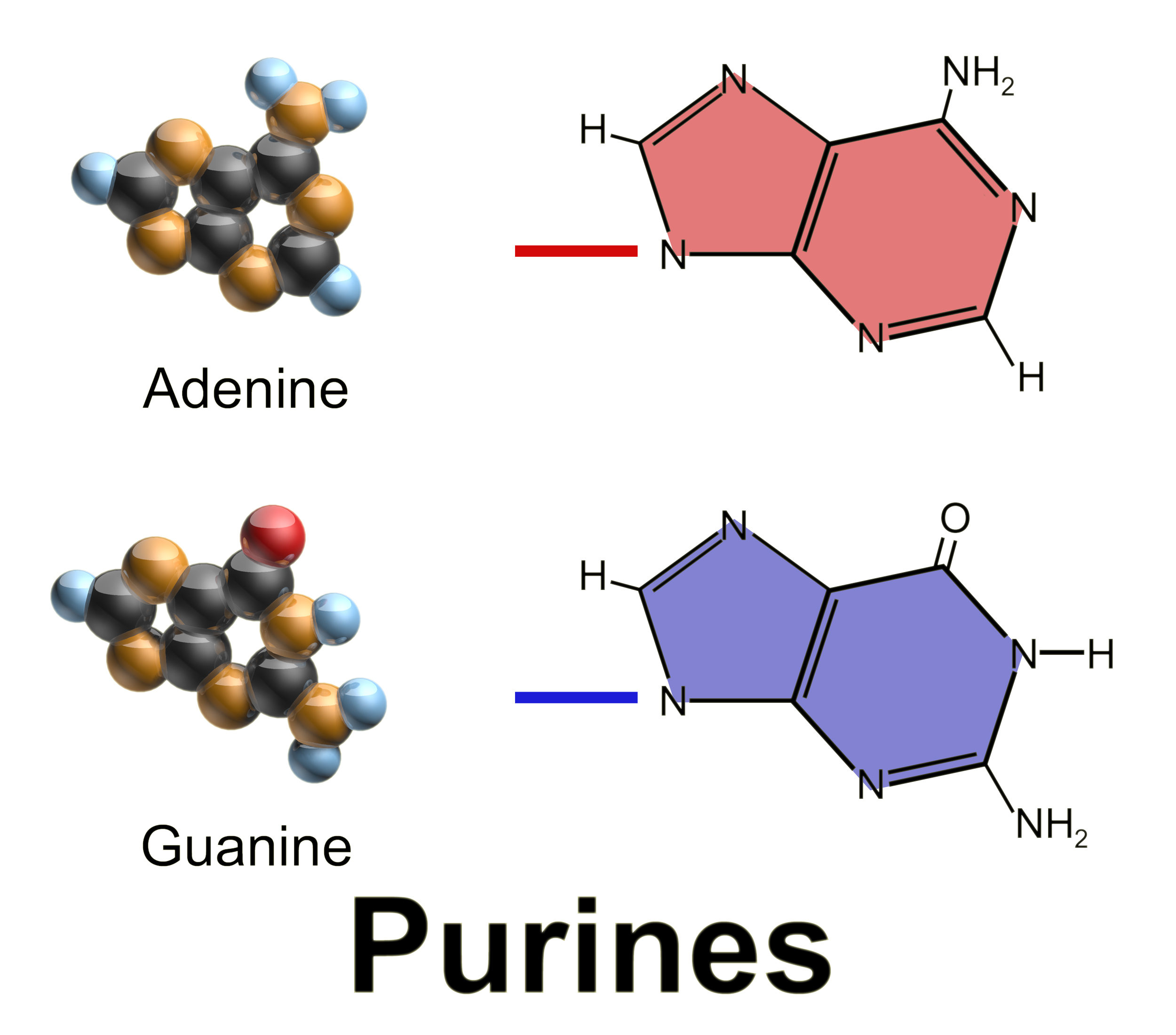
Purine
Purine is a heterocyclic aromatic organic compound that consists of two rings ( pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines and their tautomers. They are the most widely occurring nitrogen-containing heterocycles in nature. Dietary sources Purines are found in high concentration in meat and meat products, especially internal organs such as liver and kidney. In general, plant-based diets are low in purines. High-purine plants and algae include some legumes (lentils and black eye peas) and spirulina. Examples of high-purine sources include: sweetbreads, anchovies, sardines, liver, beef kidneys, brains, meat extracts (e.g., Oxo, Bovril), herring, mackerel, scallops, game meats, yeast (beer, yeast extract, nutritional yeast) and gravy. A moderate amount of purine is also contained in red meat, beef, pork, poultry, fish and seafood, asparagus, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Simple Aromatic Ring
Simple aromatic rings, also known as simple arenes or simple aromatics, are aromatic organic compounds that consist only of a conjugated planar ring system. Many simple aromatic rings have trivial names. They are usually found as substructures of more complex molecules ("substituted aromatics"). Typical simple aromatic compounds are benzene, indole, and pyridine. Simple aromatic rings can be heterocyclic if they contain non-carbon ring atoms, for example, oxygen, nitrogen, or sulfur. They can be monocyclic as in benzene, bicyclic as in naphthalene, or polycyclic as in anthracene. Simple monocyclic aromatic rings are usually five-membered rings like pyrrole or six-membered rings like pyridine. Fused/condensed aromatic rings consist of monocyclic rings that share their connecting bonds. Heterocyclic aromatic rings The nitrogen (N)-containing aromatic rings can be separated into basic aromatic rings that are easily protonated, and form aromatic cations and salts (e.g., pyridin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnoline
Cinnoline is an aromatic heterocyclic compound with the formula C8H6N2. It is isomeric with other naphthyridines including quinoxaline, phthalazine and quinazoline. Properties The free base can be obtained as an oil by treatment of the hydrochloride with base. It co-crystallizes with one molecule of ether as white silky needles, (m.p. 24–25 °C) upon cooling ethereal solutions. The free base melts at 39 °C. It has a taste resembling that of chloral hydrate and leaves a sharp irritation for some time. Cinnoline derivatives are obtained from oxycinnoline carboxylic acid, which is formed by digesting orthophenyl propiolic acid diazo chloride with water. Oxycinnoline carboxylic acid on heating gives oxycinnoline, melting at 225 °C, which with phosphorus pentachloride gives chlorcinnoline. This substance is reduced by iron filings and sulfuric acid to dihydrocinnoline. Discovery and synthesis The compound was first obtained in impure form by cyclization o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnoline Structure
Cinnoline is an aromatic heterocyclic compound with the formula C8H6N2. It is isomeric with other naphthyridines including quinoxaline, phthalazine and quinazoline. Properties The free base can be obtained as an oil by treatment of the hydrochloride with base. It co-crystallizes with one molecule of ether as white silky needles, (m.p. 24–25 °C) upon cooling ethereal solutions. The free base melts at 39 °C. It has a taste resembling that of chloral hydrate and leaves a sharp irritation for some time. Cinnoline derivatives are obtained from oxycinnoline carboxylic acid, which is formed by digesting orthophenyl propiolic acid diazo chloride with water. Oxycinnoline carboxylic acid on heating gives oxycinnoline, melting at 225 °C, which with phosphorus pentachloride gives chlorcinnoline. This substance is reduced by iron filings and sulfuric acid to dihydrocinnoline. Discovery and synthesis The compound was first obtained in impure form by cyclizati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridazine
Pyridazine is an aromatic, heterocyclic, organic compound with the molecular formula . It contains a six-membered ring with two adjacent nitrogen atoms. It is a colorless liquid with a boiling point of 208 °C. It is isomeric with two other diazine () rings, pyrimidine and pyrazine. Occurrence Pyridazines are rare in nature, possibly reflecting the scarcity of naturally occurring hydrazines, common building blocks for the synthesis of these heterocycles. The pyridazine structure is a popular pharmacophore which is found within a number of herbicides such as credazine, pyridafol and pyridate. It is also found within the structure of several drugs such as cefozopran, cadralazine, minaprine, pipofezine, and hydralazine. Synthesis In the course of his classic investigation on the Fischer indole synthesis, Emil Fischer prepared the first pyridazine via the condensation of phenylhydrazine and levulinic acid. The parent heterocycle was first prepared by oxidation of benz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridazine Structure
Pyridazine is an aromatic, heterocyclic, organic compound with the molecular formula . It contains a six-membered ring with two adjacent nitrogen atoms. It is a colorless liquid with a boiling point of 208 °C. It is isomeric with two other diazine () rings, pyrimidine and pyrazine. Occurrence Pyridazines are rare in nature, possibly reflecting the scarcity of naturally occurring hydrazines, common building blocks for the synthesis of these heterocycles. The pyridazine structure is a popular pharmacophore which is found within a number of herbicides such as credazine, pyridafol and pyridate. It is also found within the structure of several drugs such as cefozopran, cadralazine, minaprine, pipofezine, and hydralazine. Synthesis In the course of his classic investigation on the Fischer indole synthesis, Emil Fischer prepared the first pyridazine via the condensation of phenylhydrazine and levulinic acid. The parent heterocycle was first prepared by oxidation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinazoline
Quinazoline is an organic compound with the formula C8H6N2. It is an aromatic heterocycle with a bicyclic structure consisting of two fused six-membered aromatic rings, a benzene ring and a pyrimidine ring. It is a light yellow crystalline solid that is soluble in water. Also known as 1,3-diazanaphthalene, quinazoline received its name from being an aza derivative of quinoline. Though the parent quinazoline molecule is rarely mentioned by itself in technical literature, substituted derivatives have been synthesized for medicinal purposes such as antimalarial and anticancer agents. Quinazoline is a planar molecule. It is isomeric with the other diazanaphthalenes of the benzodiazine subgroup: cinnoline, quinoxaline, and phthalazine. Over 200 biologically active quinazoline and quinoline alkaloids are identified. Synthesis : The synthesis of quinazoline was first reported in 1895 by August Bischler and Lang through the decarboxylation of the 2-carboxy derivative (quinazoline-2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinazoline Structure
Quinazoline is an organic compound with the formula C8H6N2. It is an aromatic heterocycle with a bicyclic structure consisting of two fused six-membered aromatic rings, a benzene ring and a pyrimidine ring. It is a light yellow crystalline solid that is soluble in water. Also known as 1,3-diazanaphthalene, quinazoline received its name from being an aza derivative of quinoline. Though the parent quinazoline molecule is rarely mentioned by itself in technical literature, substituted derivatives have been synthesized for medicinal purposes such as antimalarial and anticancer agents. Quinazoline is a planar molecule. It is isomeric with the other diazanaphthalenes of the benzodiazine subgroup: cinnoline, quinoxaline, and phthalazine. Over 200 biologically active quinazoline and quinoline alkaloids are identified. Synthesis : The synthesis of quinazoline was first reported in 1895 by August Bischler and Lang through the decarboxylation of the 2-carboxy derivative (quinazoline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine (nitrogen atoms at the 1 and 4 positions) and pyridazine (nitrogen atoms at the 1 and 2 positions). In nucleic acids, three types of nucleobases are pyrimidine derivatives: cytosine (C), thymine (T), and uracil (U). Occurrence and history The pyrimidine ring system has wide occurrence in nature as substituted and ring fused compounds and derivatives, including the nucleotides cytosine, thymine and uracil, thiamine (vitamin B1) and alloxan. It is also found in many synthetic compounds such as barbiturates and the HIV drug, zidovudine. Although pyrimidine derivatives such as alloxan were known in the early 19th century, a laboratory synthesis of a pyrimidine was not carried out until 1879, when Grimaux reported ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrimidine Structure
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine (nitrogen atoms at the 1 and 4 positions) and pyridazine (nitrogen atoms at the 1 and 2 positions). In nucleic acids, three types of nucleobases are pyrimidine derivatives: cytosine (C), thymine (T), and uracil (U). Occurrence and history The pyrimidine ring system has wide occurrence in nature as substituted and ring fused compounds and derivatives, including the nucleotides cytosine, thymine and uracil, thiamine (vitamin B1) and alloxan. It is also found in many synthetic compounds such as barbiturates and the HIV drug, zidovudine. Although pyrimidine derivatives such as alloxan were known in the early 19th century, a laboratory synthesis of a pyrimidine was not carried out until 1879, when G ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indazole
Indazole, also called isoindazole, is a heterocyclic aromatic organic compound. This bicyclic compound consists of the fusion of benzene and pyrazole. Indazole is an amphoteric molecule which can be protonated to an indazolium cation or deprotonated to an indazolate anion. The corresponding ''pKa'' values are 1.04 for the equilibrium between indazolium cation and indazole and 13.86 for the equilibrium between indazole and indazolate anion. Indazole derivatives display a broad variety of biological activities. Indazoles are rare in nature. The alkaloids nigellicine, nigeglanine, and nigellidine are indazoles. Nigellicine was isolated from the widely distributed plant ''Nigella sativa'' L. (black cumin). Nigeglanine was isolated from extracts of ''Nigella glandulifera''. The Davis–Beirut reaction can generate 2''H''-indazoles. Indazole, C7H6N2, was obtained by E. Fischer (''Ann.'' 1883, 221, p. 280) by heating ortho-hydrazine cinnamic acid, : Some derivatives ; indazole-3- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |