|
Absolute Zero
Absolute zero is the lowest limit of the thermodynamic temperature scale, a state at which the enthalpy and entropy of a cooled ideal gas reach their minimum value, taken as zero kelvin. The fundamental particles of nature have minimum vibrational motion, retaining only quantum mechanical, zero-point energy-induced particle motion. The theoretical temperature is determined by extrapolating the ideal gas law; by international agreement, absolute zero is taken as −273.15 degrees on the Celsius scale (International System of Units), Note: The triple point of water is 0.01 °C, not 0 °C; thus 0 K is −2890.15 °C, not −273.16 °C. which equals −459.67 degrees on the Fahrenheit scale ( United States customary units or Imperial units). The corresponding Kelvin and Rankine temperature scales set their zero points at absolute zero by definition. It is commonly thought of as the lowest temperature possible, but it is not the lowest ''enthalpy'' state poss ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ground State
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. In quantum field theory, the ground state is usually called the vacuum state or the vacuum. If more than one ground state exists, they are said to be degenerate. Many systems have degenerate ground states. Degeneracy occurs whenever there exists a unitary operator that acts non-trivially on a ground state and commutes with the Hamiltonian of the system. According to the third law of thermodynamics, a system at absolute zero temperature exists in its ground state; thus, its entropy is determined by the degeneracy of the ground state. Many systems, such as a perfect crystal lattice, have a unique ground state and therefore have zero entropy at absolute zero. It is also possible for the highest excited state to have absolute zero temper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Walther Nernst
Walther Hermann Nernst (; 25 June 1864 – 18 November 1941) was a German chemist known for his work in thermodynamics, physical chemistry, electrochemistry, and solid state physics. His formulation of the Nernst heat theorem helped pave the way for the third law of thermodynamics, for which he won the 1920 Nobel Prize in Chemistry. He is also known for developing the Nernst equation in 1887. Life and career Early years Nernst was born in Briesen in West Prussia (now Wąbrzeźno, Poland) to Gustav Nernst (1827–1888) and Ottilie Nerger (1833–1876). His father was a country judge. Nernst had three older sisters and one younger brother. His third sister died of cholera. Nernst went to elementary school at Graudenz. He studied physics and mathematics at the universities of Zürich, Berlin, Graz and Würzburg, where he received his doctorate 1887. In 1889, he finished his habilitation at University of Leipzig. Personal attributes It was said that Nernst was mechanically minde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Third Law Of Thermodynamics
The third law of thermodynamics states, regarding the properties of closed systems in thermodynamic equilibrium: This constant value cannot depend on any other parameters characterizing the closed system, such as pressure or applied magnetic field. At absolute zero (zero kelvins) the system must be in a state with the minimum possible energy. Entropy is related to the number of accessible microstates, and there is typically one unique state (called the ground state) with minimum energy. In such a case, the entropy at absolute zero will be exactly zero. If the system does not have a well-defined order (if its order is glassy, for example), then there may remain some finite entropy as the system is brought to very low temperatures, either because the system becomes locked into a configuration with non-minimal energy or because the minimum energy state is non-unique. The constant value is called the residual entropy of the system. The entropy is essentially a state-function meaning t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Max Planck
Max Karl Ernst Ludwig Planck (, ; 23 April 1858 – 4 October 1947) was a German theoretical physicist whose discovery of energy quanta won him the Nobel Prize in Physics in 1918. Planck made many substantial contributions to theoretical physics, but his fame as a physicist rests primarily on his role as the originator of quantum theory, which revolutionized human understanding of atomic and subatomic processes. In 1948, the German scientific institution Kaiser Wilhelm Society (of which Planck was twice president) was renamed Max Planck Society (MPG). The MPG now includes 83 institutions representing a wide range of scientific directions. Life and career Planck came from a traditional, intellectual family. His paternal great-grandfather and grandfather were both theology professors in Göttingen; his father was a law professor at the University of Kiel and Munich. One of his uncles was also a judge. Planck was born in 1858 in Kiel, Holstein, to Johann Julius Wilhelm Plan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfect Crystal
Crystalline materials (mainly metals and alloys, but also stoichiometric salts and other materials) are made up of solid regions of ordered matter (atoms placed in one of a number of ordered formations called Bravais lattices). These regions are known as crystals. A perfect crystal is a crystal that contains no point, line, or planar defects. There are a wide variety of crystallographic defects. The hypothetical concept of a perfect crystal is important in the basic formulation of the third law of thermodynamics. In crystallography, the phrase 'perfect crystal' can be used to mean "no linear or planar imperfections", as it is difficult to measure small quantities of point imperfections in an otherwise defect-free crystal. Imperfections are created by various thermodynamic Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adiabatic Process
In thermodynamics, an adiabatic process (Greek: ''adiábatos'', "impassable") is a type of thermodynamic process that occurs without transferring heat or mass between the thermodynamic system and its environment. Unlike an isothermal process, an adiabatic process transfers energy to the surroundings only as work.. A translation may be founhere. Also a mostly reliabltranslation is to be foundin As a key concept in thermodynamics, the adiabatic process supports the theory that explains the first law of thermodynamics. Some chemical and physical processes occur too rapidly for energy to enter or leave the system as heat, allowing a convenient "adiabatic approximation".Bailyn, M. (1994), pp. 52–53. For example, the adiabatic flame temperature uses this approximation to calculate the upper limit of flame temperature by assuming combustion loses no heat to its surroundings. In meteorology and oceanography, adiabatic cooling produces condensation of moisture or salinity, oversatu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superfluidity
Superfluidity is the characteristic property of a fluid with zero viscosity which therefore flows without any loss of kinetic energy. When stirred, a superfluid forms vortices that continue to rotate indefinitely. Superfluidity occurs in two isotopes of helium (helium-3 and helium-4) when they are liquefied by cooling to cryogenic temperatures. It is also a property of various other exotic State of matter, states of matter theorized to exist in astrophysics, high-energy physics, and theories of quantum gravity. The theory of superfluidity was developed by Soviet theoretical physicists Lev Landau and Isaak Khalatnikov. Superfluidity is often coincidental with Bose–Einstein condensate, Bose–Einstein condensation, but neither phenomenon is directly related to the other; not all Bose–Einstein condensates can be regarded as superfluids, and not all superfluids are Bose–Einstein condensates. Superfluidity of liquid helium Superfluidity was discovered in helium-4 by Pyotr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superconductivity
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike an ordinary metallic conductor, whose resistance decreases gradually as its temperature is lowered even down to near absolute zero, a superconductor has a characteristic critical temperature below which the resistance drops abruptly to zero. An electric current through a loop of superconducting wire can persist indefinitely with no power source. The superconductivity phenomenon was discovered in 1911 by Dutch physicist Heike Kamerlingh Onnes. Like ferromagnetism and atomic spectral lines, superconductivity is a phenomenon which can only be explained by quantum mechanics. It is characterized by the Meissner effect, the complete ejection of magnetic field lines from the interior of the superconductor during its transitions into the sup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
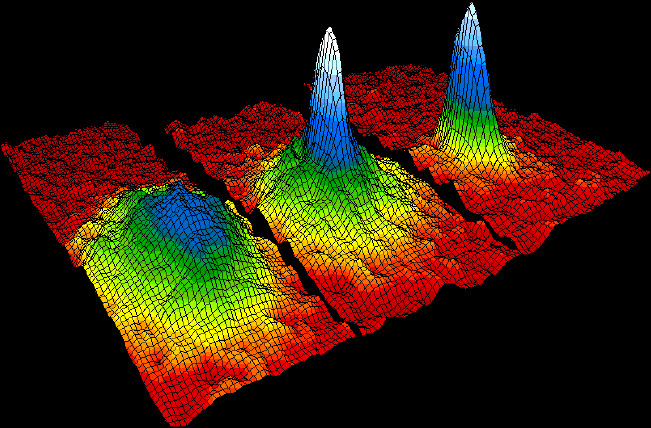
Bose–Einstein Condensate
In condensed matter physics, a Bose–Einstein condensate (BEC) is a state of matter that is typically formed when a gas of bosons at very low densities is cooled to temperatures very close to absolute zero (−273.15 °C or −459.67 °F). Under such conditions, a large fraction of bosons occupy the lowest quantum state, at which point microscopic quantum mechanical phenomena, particularly wavefunction interference, become apparent macroscopically. A BEC is formed by cooling a gas of extremely low density (about 100,000 times less dense than normal air) to ultra-low temperatures. This state was first predicted, generally, in 1924–1925 by Albert Einstein following and crediting a pioneering paper by Satyendra Nath Bose on the new field now known as quantum statistics. In 1995, the Bose-Einstein condensate was created by Eric Cornell and Carl Wieman of the University of Colorado at Boulder using rubidium atoms; later that year, Wolfgang Ketterle of MIT produc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, quantum field theory, quantum technology, and quantum information science. Classical physics, the collection of theories that existed before the advent of quantum mechanics, describes many aspects of nature at an ordinary (macroscopic) scale, but is not sufficient for describing them at small (atomic and subatomic) scales. Most theories in classical physics can be derived from quantum mechanics as an approximation valid at large (macroscopic) scale. Quantum mechanics differs from classical physics in that energy, momentum, angular momentum, and other quantities of a bound system are restricted to discrete values ( quantization); objects have characteristics of both particles and waves (wave–particle duality); and there are limits to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinetic Energy
In physics, the kinetic energy of an object is the energy that it possesses due to its motion. It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes. The same amount of work is done by the body when decelerating from its current speed to a state of rest. Formally, a kinetic energy is any term in a system's Lagrangian which includes a derivative with respect to time. In classical mechanics, the kinetic energy of a non-rotating object of mass ''m'' traveling at a speed ''v'' is \fracmv^2. In relativistic mechanics, this is a good approximation only when ''v'' is much less than the speed of light. The standard unit of kinetic energy is the joule, while the English unit of kinetic energy is the foot-pound. History and etymology The adjective ''kinetic'' has its roots in the Greek word κίνησις ''kinesis'', m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |