|
Annihilation Radiation
Annihilation radiation is a term used in Gamma spectroscopy for the photon radiation produced when a particle and its antiparticle collide and annihilate. Most commonly, this refers to 511-k eV photons produced by an electron interacting with a positron. These photons are frequently referred to as gamma rays, despite having their origin outside the nucleus, due to unclear distinctions between types of photon radiation. The positively-charged antiparticle of the electron (known as the positron) is emitted from the nucleus as it undergoes β+ decay. The positron travels a short distance (a few millimeters), depositing any excess energy before it combines with a free electron. The mass of the e- and e+ is completely converted into two photons with an energy of 511 keV each. These annihilation photons are emitted in opposite directions, 180˚ apart. This is the basis for PET scanners in a process called coincidence counting. Annihilation radiation is not monoenergetic, unlike gamma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Gamma Spectroscopy
Gamma-ray spectroscopy is the ''qualitative'' study of the energy spectra of gamma-ray sources, such as in the nuclear industry, geochemical investigation, and astrophysics. Gamma-ray spectrometry, on the other hand, is the method used to acquire a ''quantitative'' spectrum measurement. Most radioactive sources produce gamma rays, which are of various energies and intensities. When these emissions are detected and analyzed with a spectroscopy system, a gamma-ray energy spectrum can be produced. A detailed analysis of this spectrum is typically used to determine the identity and quantity of gamma emitters present in a gamma source, and is a vital tool in radiometric assay. The gamma spectrum is characteristic of the gamma-emitting nuclides contained in the source, just like in an optical spectrometer, the optical spectrum is characteristic of the material contained in a sample. Gamma ray characteristics Gamma rays are the highest-energy form of electromagnetic radiation, being ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Annihilation Radiation
Annihilation radiation is a term used in Gamma spectroscopy for the photon radiation produced when a particle and its antiparticle collide and annihilate. Most commonly, this refers to 511-k eV photons produced by an electron interacting with a positron. These photons are frequently referred to as gamma rays, despite having their origin outside the nucleus, due to unclear distinctions between types of photon radiation. The positively-charged antiparticle of the electron (known as the positron) is emitted from the nucleus as it undergoes β+ decay. The positron travels a short distance (a few millimeters), depositing any excess energy before it combines with a free electron. The mass of the e- and e+ is completely converted into two photons with an energy of 511 keV each. These annihilation photons are emitted in opposite directions, 180˚ apart. This is the basis for PET scanners in a process called coincidence counting. Annihilation radiation is not monoenergetic, unlike gamma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Sodium Iodide
Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions (I−) in a crystal lattice. It is used mainly as a nutritional supplement and in organic chemistry. It is produced industrially as the salt formed when acidic iodides react with sodium hydroxide. It is a chaotropic salt. Uses Food supplement Sodium iodide, as well as potassium iodide, is commonly used to treat and prevent iodine deficiency. Iodized table salt contains 10 ppm iodide. Organic synthesis Sodium iodide is used for conversion of alkyl chlorides into alkyl iodides. This method, the Finkelstein reaction, relies on the insolubility of sodium chloride in acetone to drive the reaction: ::R–Cl + NaI → R–I + NaCl Nuclear medicine Some radioactive iodide salts of sodium, including Na 125I and Na 131I, have rad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Germanium
Germanium is a chemical element; it has Symbol (chemistry), symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid or a nonmetal in the carbon group that is chemically similar to silicon. Like silicon, germanium naturally Chemical reaction, reacts and forms complexes with oxygen in nature. Because it seldom appears in high concentration, germanium was found comparatively late in the Timeline of chemical element discoveries, discovery of the elements. Germanium ranks 50th Abundance of elements in Earth's crust, in abundance of the elements in the Earth's crust. In 1869, Dmitri Mendeleev Mendeleev's predicted elements, predicted its existence and some of its Chemical property, properties from its position on his periodic table, and called the element ekasilicon. On February 6, 1886, Clemens Winkler at Freiberg University found the new element, along with silver and sulfur, in the mineral argyrodite. Winkle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Full Width At Half Maximum
In a distribution, full width at half maximum (FWHM) is the difference between the two values of the independent variable at which the dependent variable is equal to half of its maximum value. In other words, it is the width of a spectrum curve measured between those points on the ''y''-axis which are half the maximum amplitude. Half width at half maximum (HWHM) is half of the FWHM if the function is symmetric. The term full duration at half maximum (FDHM) is preferred when the independent variable is time. FWHM is applied to such phenomena as the duration of pulse waveforms and the spectral width of sources used for optical communications and the resolution of spectrometers. The convention of "width" meaning "half maximum" is also widely used in signal processing to define bandwidth as "width of frequency range where less than half the signal's power is attenuated", i.e., the power is at least half the maximum. In signal processing terms, this is at most −3 dB of att ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Gerard F
Gerard is a masculine forename of Proto-Germanic origin, variations of which exist in many Germanic and Romance languages. Like many other early Germanic names, it is dithematic, consisting of two meaningful constituents put together. In this case, those constituents are ''gari'' > ''ger-'' (meaning 'spear') and -''hard'' (meaning 'hard/strong/brave'). Common forms of the name are Gerard (English, Scottish, Irish, Dutch, Polish and Catalan); Gerrard (English, Scottish, Irish); (Italian, and Spanish); ( Portuguese); (Italian); (Northern Italian, now only a surname); (variant forms and , now only surnames, French); ( Irish); Gerhardt and Gerhart/Gerhard/Gerhardus (German, Dutch, and Afrikaans); ( Hungarian); ( Lithuanian) and / ( Latvian); (Greece). A few abbreviated forms are Gerry and Jerry (English); (German) and (Afrikaans and Dutch); (Afrikaans and Dutch); (Afrikaans); (Dutch) and ( Bulgarian). The introduction of the name 'Gerard' into the English language ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
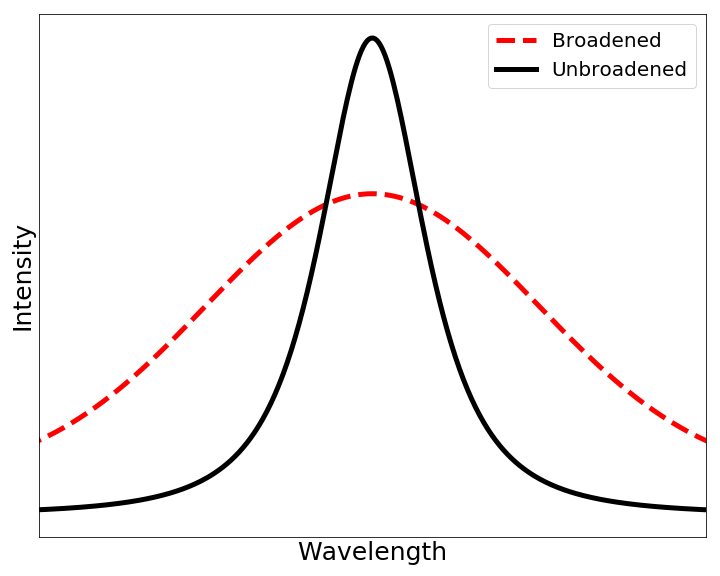
Doppler Broadening
In atomic physics, Doppler broadening is broadening of spectral lines due to the Doppler effect caused by a distribution of velocities of atoms or molecules. Different velocities of the emitting (or absorbing) particles result in different Doppler shifts, the cumulative effect of which is the emission (absorption) line broadening. This resulting line profile is known as a Doppler profile. A particular case is the thermal Doppler broadening due to the thermal motion of the particles. Then, the broadening depends only on the frequency of the spectral line, the mass of the emitting particles, and their temperature, and therefore can be used for inferring the temperature of an emitting (or absorbing) body being spectroscopically investigated. Derivation (non-relativistic case) When a particle moves (e.g., due to the thermal motion) towards the observer, the emitted radiation is shifted to a higher frequency. Likewise, when the emitter moves away, the frequency is lowered. In th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Radioactive Decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered ''radioactive''. Three of the most common types of decay are Alpha decay, alpha, Beta decay, beta, and Gamma ray, gamma decay. The weak force is the Fundamental interactions, mechanism that is responsible for beta decay, while the other two are governed by the electromagnetic force, electromagnetic and nuclear forces. Radioactive decay is a randomness, random process at the level of single atoms. According to quantum mechanics, quantum theory, it is impossible to predict when a particular atom will decay, regardless of how long the atom has existed. However, for a significant number of identical atoms, the overall decay rate can be expressed as a decay constant or as a half-life. The half-lives of radioactive atoms have a huge range: f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Positron Emission Tomography
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body, such as: * Fluorodeoxyglucose ( 18F">sup>18FDG or FDG) is commonly used to detect cancer; * 18Fodium fluoride">sup>18Fodium fluoride (Na18F) is widely used for detecting bone formation; * Oxygen-15 (15O) is sometimes used to measure blood flow. PET is a common imaging technique, a medical scintillography technique used in nuclear medicine. A radiopharmaceutical—a radioisotope attached to a drug—is injected into the body as a tracer. When the radiopharmaceutical undergoes beta plus decay, a positron is emitted, and when the positron interacts with an ordinary electron, the tw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Elementary Particle
In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles. The Standard Model presently recognizes seventeen distinct particles—twelve fermions and five bosons. As a consequence of flavor and color combinations and antimatter, the fermions and bosons are known to have 48 and 13 variations, respectively. Among the 61 elementary particles embraced by the Standard Model number: electrons and other leptons, quarks, and the fundamental bosons. Subatomic particles such as protons or neutrons, which contain two or more elementary particles, are known as composite particles. Ordinary matter is composed of atoms, themselves once thought to be indivisible elementary particles. The name ''atom'' comes from the Ancient Greek word ''ἄτομος'' ( atomos) which means ''indivisible'' or ''uncuttable''. Despite the theories about atoms that had existed for thousands of years, the factual existence of ato ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
X-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ranging from 10 Nanometre, nanometers to 10 Picometre, picometers, corresponding to frequency, frequencies in the range of 30 Hertz, petahertz to 30 Hertz, exahertz ( to ) and photon energies in the range of 100 electronvolt, eV to 100 keV, respectively. X-rays were discovered in 1895 in science, 1895 by the German scientist Wilhelm Röntgen, Wilhelm Conrad Röntgen, who named it ''X-radiation'' to signify an unknown type of radiation.Novelline, Robert (1997). ''Squire's Fundamentals of Radiology''. Harvard University Press. 5th edition. . X-rays can penetrate many solid substances such as construction materials and living tissue, so X-ray radiography is widely used in medical diagnostics (e.g., checking for Bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Gamma Ray
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz () and wavelengths less than 10 picometers (), gamma ray photons have the highest photon energy of any form of electromagnetic radiation. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation ''gamma rays'' based on their relatively strong penetration of matter; in 1900, he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power. Gamma rays from radioactive decay are in the energy range ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |