|
Tungsten(VI) Oxide
Tungsten(VI) oxide, also known as tungsten trioxide is a chemical compound of oxygen and the transition metal tungsten, with formula WO3. The compound is also called tungstic anhydride, reflecting its relation to tungstic acid . It is a light yellow crystalline solid. Tungsten(VI) oxide occurs naturally in the form of hydrates, which include minerals: tungstite WO3·H2O, meymacite WO3·2H2O and hydrotungstite (of the same composition as meymacite, however sometimes written as H2WO4). These minerals are rare to very rare secondary tungsten minerals. History In 1841, a chemist named Robert Oxland gave the first procedures for preparing tungsten trioxide and sodium tungstate. He was granted patents for his work soon after, and is considered to be the founder of systematic tungsten chemistry. Structure and properties The crystal structure of tungsten trioxide is temperature dependent. It is tetragonal at temperatures above 740 °C, orthorhombic from 330 to 740 °C, monoclini ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluoric Acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colourless, acidic and highly corrosive. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers. Uses Production of organofluorine compounds The principal use of hydrofluoric acid is in organofluorine chemistry. Many organofluorine compounds are prepared using HF as the fluorine source, including Teflon, fluoropolymers, fluorocarbons, and refrigerants such as freon. Many pharmaceuticals contain fluorine. Production of inorganic fluorides Most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost are Na3AlF6, cryolite, and AlF3, aluminium trifluoride. A molten mixture of these solids serves as a high-temperature solvent for the prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robert Oxland
Air Vice Marshal Robert Dickinson Oxland, (4 April 1889 – 27 October 1959) was a senior Royal Air Force officer and member of Bomber Command during the Second World War.''Kelly’s Handbook to the Titled, Landed and Official Classes 1958'', Published by Kelly’s Directories Limited, 1958 He was air officer commanding No. 1 Group from 1940 to 1943. Early life Robert Dickinson Oxland was born in Sydenham on 4 April 1889, the son of Charles Oxland, a Mining Engineer, and his wife Eleanor. He was educated at Bedford Modern School. Career At the outbreak of the First World War, Oxland joined the County of London Yeomanry.The Times, 23 October 1934 He was commissioned in 1915 and seconded to the Royal Flying Corps in 1916 having learned to fly in Norwich, earning RAeC Certificate No. 2444 on 9 February 1916. He was with No. 20 Squadron in France in 1916 and with No. 38 Squadron in 1918. Oxland transferred to the Royal Air Force in 1918. As a qualified meteorological observe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
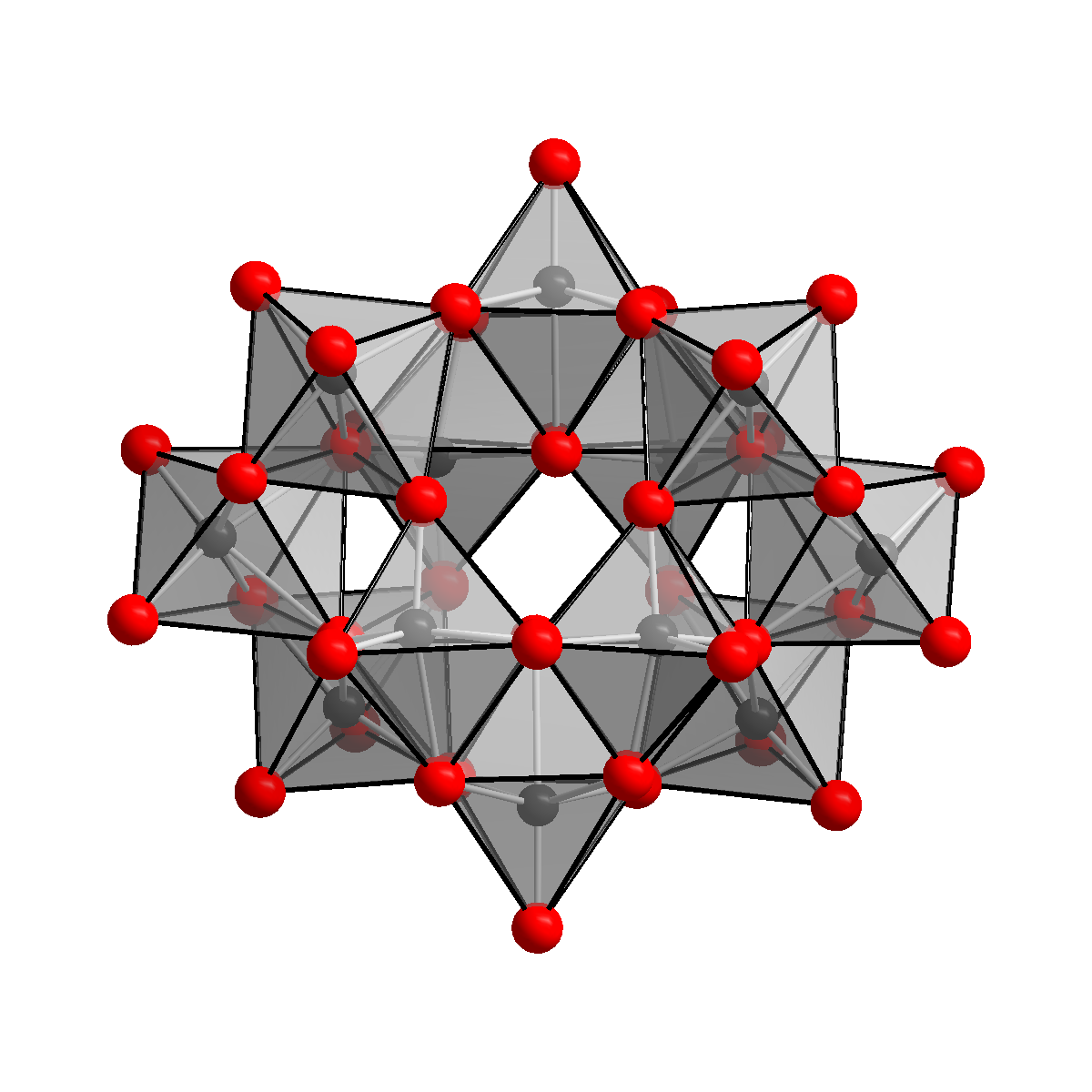
Ammonium Paratungstate
Ammonium paratungstate (or APT) is a white crystalline salt with the chemical formula (NH4)10(H2W12O42)·4H2O. It is described as "the most important raw material for all other tungsten products." Production From tungsten ores Tungsten ores, which are typically oxides, are digested in base to give solutions of tungstate together with many contaminating species. This crude extract is acidified and treated with sulfide to separate molybdenum trisulfide. Upon further acidification APT eventually crystallizes. Laboratory methods If a calcined WO3 is used, refluxing the ammonia solution is advisable to accelerate its dissolution. Conversion to tungsten metal Heating ammonium paratungstate to its decomposition temperature of 600 °C yields tungsten(VI) oxide, as described in this idealized equation: :(NH4)10(H2W12O42)·4H2O → 12 WO3 + 10 NH3 + 6 H2O From there, the trioxide is heated in an atmosphere of hydrogen, yielding elemental tungsten: :WO3 + 3 H2 → W + 3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcination
Calcination refers to thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally for the purpose of removing impurities or volatile substances and/or to incur thermal decomposition. The root of the word calcination refers to its most prominent use, which is to remove carbon from limestone (calcium carbonate) through combustion to yield calcium oxide (quicklime). This calcination reaction is CaCO3(s) → CaO(s) + CO2(g). Calcium oxide is a crucial ingredient in modern cement, and is also used as a chemical flux in smelting. Industrial calcination generally emits carbon dioxide (), making it a major contributor to climate change. A calciner is a steel cylinder that rotates inside a heated furnace and performs indirect high-temperature processing (550–1150 °C, or 1000–2100 °F) within a controlled ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scheelite
Scheelite is a calcium tungstate mineral with the chemical formula Ca W O4. It is an important ore of tungsten (wolfram). Scheelite is originally named after Swedish chemist K. Scheele (1742-1786). Well-formed crystals are sought by collectors and are occasionally fashioned into gemstones when suitably free of flaws. Scheelite has been synthesized using the Czochralski process; the material produced may be used to imitate diamond, as a scintillator, or as a solid-state lasing medium. It was also used in radium paint in the same fashion as was zinc sulphide, and Thomas Edison invented a fluoroscope with a calcium tungstate-coated screen, making the images six times brighter than those with barium platinocyanide; the latter chemical allowed Röntgen to discover X-rays in early November 1895. Properties Its crystals are in the tetragonal crystal system, appearing as dipyramidal pseudo-octahedra. Colors include golden yellow, brownish green to dark brown, pinkish to reddis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungstate
In chemistry, a tungstate is a compound that contains an oxyanion of tungsten or is a mixed oxide containing tungsten. The simplest tungstate ion is , "orthotungstate". Many other tungstates belong to a large group of polyatomic ions that are termed polyoxometalates, ("POMs"), and specifically termed isopolyoxometalates as they contain, along with oxygen and maybe hydrogen, only one other element. Almost all useful tungsten ores are tungstates. Structures Orthotungstates feature tetrahedral W(VI) centres with short W–O distances of 1.79 Å. Structurally, they resemble sulfates. Six-coordinate, octahedral tungsten dominates in the polyoxotungstates. In these compounds, the W–O distances are elongated. Some examples of tungstate ions: * (hydrogentungstate) * polymeric ions of various structures in , and Wells A.F. (1984) ''Structural Inorganic Chemistry'' 5th edition Oxford Science Publications * (paratungstate A) * (tungstate Y)Jon A. McCleverty, N. G. Connelly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The adjective alkaline, and less often, alkalescent, is commonly used in English as a synonym for basic, especially for bases soluble in water. This broad use of the term is likely to have come about because alkalis were the first bases known to obey the Arrhenius definition of a base, and they are still among the most common bases. Etymology The word "alkali" is derived from Arabic ''al qalīy'' (or ''alkali''), meaning ''the calcined ashes'' (see calcination), referring to the original source of alkaline substances. A water-extract of burned plant ashes, called potash and composed mostly of potassium carbonate, was mildly basic. After heating this substance with calcium hydroxide (''slaked lim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superconductivity
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike an ordinary metallic conductor, whose resistance decreases gradually as its temperature is lowered even down to near absolute zero, a superconductor has a characteristic critical temperature below which the resistance drops abruptly to zero. An electric current through a loop of superconducting wire can persist indefinitely with no power source. The superconductivity phenomenon was discovered in 1911 by Dutch physicist Heike Kamerlingh Onnes. Like ferromagnetism and atomic spectral lines, superconductivity is a phenomenon which can only be explained by quantum mechanics. It is characterized by the Meissner effect, the complete ejection of magnetic field lines from the interior of the superconductor during its transitions into t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungsten Dioxide
Tungsten(IV) oxide is the chemical compound with the formula W O2. The bronze-colored solid crystallizes in a monoclinic cell. The rutile Rutile is an oxide mineral composed of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer polymorphs of TiO2 are known, including anatase, akaogiite, and brookite. Rutile has one of the highest refractive indices at visib ...-like structure features distorted octahedral WO6 centers with alternate short W–W bonds (248 pm). Each tungsten center has the d2 configuration, which gives the material a high electrical conductivity. WO2 is prepared by reduction of WO3 with tungsten powder over the course of 40 hours at 900 °C. An intermediate in this reaction is the partially reduced, mixed valence species W18O49. :2 WO3 + W → 3 WO2 The molybdenum analogue MoO2 is prepared similarly. Single crystals are obtained by chemical transport technique using iodine. Iodine transports the WO2 in the fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Space Group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchanged. In three dimensions, space groups are classified into 219 distinct types, or 230 types if chiral copies are considered distinct. Space groups are discrete cocompact groups of isometries of an oriented Euclidean space in any number of dimensions. In dimensions other than 3, they are sometimes called Bieberbach groups. In crystallography, space groups are also called the crystallographic or Fedorov groups, and represent a description of the symmetry of the crystal. A definitive source regarding 3-dimensional space groups is the ''International Tables for Crystallography'' . History Space groups in 2 dimensions are the 17 wallpaper groups which have been known for several centuries, though the proof that the list was complete was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triclinic
180px, Triclinic (a ≠ b ≠ c and α ≠ β ≠ γ ) In crystallography, the triclinic (or anorthic) crystal system is one of the 7 crystal systems. A crystal system is described by three basis vectors. In the triclinic system, the crystal is described by vectors of unequal length, as in the orthorhombic system. In addition, the angles between these vectors must all be different and may not include 90°. The triclinic lattice is the least symmetric of the 14 three-dimensional Bravais lattices. It has (itself) the minimum symmetry all lattices have: points of inversion at each lattice point and at 7 more points for each lattice point: at the midpoints of the edges and the faces, and at the center points. It is the only lattice type that itself has no mirror planes. Crystal classes The triclinic crystal system class names, examples, Schönflies notation, Hermann-Mauguin notation, point groups, International Tables for Crystallography space group number, orbifold, type, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic system. They form a parallelogram prism. Hence two pairs of vectors are perpendicular (meet at right angles), while the third pair makes an angle other than 90°. Bravais lattices Two monoclinic Bravais lattices exist: the primitive monoclinic and the base-centered monoclinic. For the base-centered monoclinic lattice, the primitive cell has the shape of an oblique rhombic prism;See , row mC, column Primitive, where the cell parameters are given as a1 = a2, α = β it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. Note that the length a of the primitive cell below equals \frac \sqrt of the conventional cell above. Crystal classes The table below org ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_0468.jpg)