|
Tert-butylthiol
''tert''-Butylthiol, also known as 2-methylpropane-2-thiol, 2-methyl-2-propanethiol, ''tert''-butyl mercaptan (TBM), and ''t''-BuSH, is an organosulfur compound with the formula (CH3)3CSH. This thiol is used as an odorant for natural gas, which is otherwise odorless. It may also have been used as a flavoring agent. Preparation At least one publication has listed ''tert''-butylthiol as a very minor component of cooked potatoes, but because the ''tert''-butyl moiety is very rare in natural products, other sources doubt the existence of natural sources of the compound. It was first prepared in 1890 by Leonard Dobbin by the reaction of zinc sulfide and ''t''-butyl chloride. The compound was later prepared in 1932 by the reaction of the Grignard reagent, ''t''-BuMgCl, with sulfur to give the corresponding thiolate, followed by hydrolysis. This preparation is shown below: :''t''-BuMgCl + S → ''t''-BuSMgCl :''t''-BuSMgCl + H2O → ''t''-BuSH + Mg(OH)Cl It is currentl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant. Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate group () bonds very strong ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
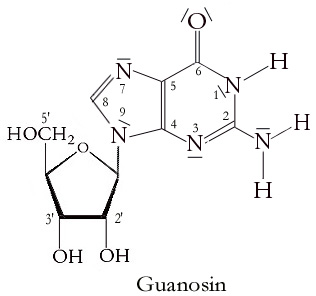
Guanosine
Guanosine (symbol G or Guo) is a purine nucleoside comprising guanine attached to a ribose (ribofuranose) ring via a β-N9-glycosidic bond. Guanosine can be phosphorylated to become guanosine monophosphate (GMP), cyclic guanosine monophosphate (cGMP), guanosine diphosphate (GDP), and guanosine triphosphate (GTP). These forms play important roles in various biochemical processes such as synthesis of nucleic acids and proteins, photosynthesis, muscle contraction, and intracellular signal transduction (cGMP). When guanine is attached by its N9 nitrogen to the C1 carbon of a deoxyribose ring it is known as deoxyguanosine. Physical and chemical properties Guanosine is a white, crystalline powder with no odor and mild saline taste. It is very soluble in acetic acid, slightly soluble in water, insoluble in ethanol, diethyl ether, benzene and chloroform. Functions Guanosine is required for an RNA splicing reaction in mRNA, when a "self-splicing" intron removes itself from the mRNA messag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butanethiol
1-Butanethiol, also known as butyl mercaptan, is a volatile, clear to yellowish liquid with a fetid (extremely foul-smelling) odor, commonly described as "skunk" odor. In fact, 1-butanethiol is structurally similar to several major constituents of a skunk's defensive spray but is not actually present in the spray. The scent of 1-butanethiol is so strong that the human nose can easily detect it in the air at concentrations as low as 10 parts per billion. The threshold level for 1-butanethiol is reported as 1.4 ppb Chemistry 1-Butanethiol is chemically classified among the thiols, which are organic compounds with molecular formulas and structural formulas similar to alcohols, except that the sulfur-containing sulfhydryl group (-SH) replaces the oxygen-containing hydroxyl group (-OH) in the molecule. 1-Butanethiol's basic molecular formula is C4H9SH, and its structural formula is similar to that of the alcohol ''n''-butanol. 1-Butanethiol is prepared by the free radical catalyzed ad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Food Safety Authority
The European Food Safety Authority (EFSA) is the agency of the European Union (EU) that provides independent scientific advice and communicates on existing and emerging risks associated with the food chain. EFSA was established in February 2002, is based in Parma, Italy, and for 2021 it has a budget of €118.6 million, and a total staff of 542. The work of EFSA covers all matters with a direct or indirect impact on food and feed safety, including animal health and welfare, plant protection and plant health and nutrition. EFSA supports the European Commission, the European Parliament and EU member states in taking effective and timely risk management decisions that ensure the protection of the health of European consumers and the safety of the food and feed chain. EFSA also communicates to the public in an open and transparent way on all matters within its remit. Structure Based on a regulation of 2002, the EFSA is composed of four bodies: * Management Board * Executive Dir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butanethiol
1-Butanethiol, also known as butyl mercaptan, is a volatile, clear to yellowish liquid with a fetid (extremely foul-smelling) odor, commonly described as "skunk" odor. In fact, 1-butanethiol is structurally similar to several major constituents of a skunk's defensive spray but is not actually present in the spray. The scent of 1-butanethiol is so strong that the human nose can easily detect it in the air at concentrations as low as 10 parts per billion. The threshold level for 1-butanethiol is reported as 1.4 ppb Chemistry 1-Butanethiol is chemically classified among the thiols, which are organic compounds with molecular formulas and structural formulas similar to alcohols, except that the sulfur-containing sulfhydryl group (-SH) replaces the oxygen-containing hydroxyl group (-OH) in the molecule. 1-Butanethiol's basic molecular formula is C4H9SH, and its structural formula is similar to that of the alcohol ''n''-butanol. 1-Butanethiol is prepared by the free radical catalyzed ad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sec-Butyl Mercaptan
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane. The isomer ''n''-butane can connect in two ways, giving rise to two "-butyl" groups: * If it connects at one of the two terminal carbon atoms, it is normal butyl or ''n''-butyl: (preferred IUPAC name: butyl) * If it connects at one of the non-terminal (internal) carbon atoms, it is secondary butyl or ''sec''-butyl: (preferred IUPAC name: butan-2-yl) The second isomer of butane, isobutane, can also connect in two ways, giving rise to two additional groups: * If it connects at one of the three terminal carbons, it is isobutyl: (preferred IUPAC name: 2-methylpropyl) * If it connects at the central carbon, it is tertiary butyl, ''tert''-butyl or ''t''-butyl: (preferred IUPAC name: ''tert''-butyl) Nomenclature According to IUPAC nomenclature, "isobutyl", "''sec''-butyl", and "''tert''-b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropyl Mercaptan
In organic chemistry, propyl is a three-carbon alkyl substituent with chemical formula for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent is often represented in organic chemistry with the symbol Pr (not to be confused with the element praseodymium). An isomeric form of propyl is obtained by moving the point of attachment from a terminal carbon atom to the central carbon atom, named 1-methylethyl or isopropyl. To maintain four substituents on each carbon atom, one hydrogen atom has to be moved from the middle carbon atom to the carbon atom which served as attachment point in the ''n''-propyl variant, written as . Linear propyl is sometimes termed normal and hence written with a prefix ''n''- (i.e., ''n-''propyl), as the absence of the prefix ''n''- does not indicate which attachment point is chosen, i.e. absence of prefix does not automatically exclude the possibility of it being ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrothiophene
Tetrahydrothiophene is an organosulfur compound with the formula (CH2)4S. The molecule consists of a five-membered saturated ring with four methylene groups and a sulfur atom. It is the saturated analog of thiophene. It is a volatile, colorless liquid with an intensely unpleasant odor. It is also known as thiophane, thiolane, or THT. While THT is not particularly common, the vitamin biotin is essential for life in aerobic organisms. Synthesis and reactions Tetrahydrothiophene is prepared by the reaction of tetrahydrofuran with hydrogen sulfide. This vapor-phase reaction is catalyzed by alumina and other heterogenous acid catalysts. This compound is a ligand in coordination chemistry, an example being the complex chloro(tetrahydrothiophene)gold(I). Oxidation of THT gives the sulfone sulfolane, which is of interest as a polar, odorless solvent: : Sulfolane is, however, more conventionally prepared from butadiene. Natural occurrence Both unsubstituted and substituted tetra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Ethyl Sulfide
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For example, protonation of methanol gives an electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyl Sulfide
Dimethyl sulfide (DMS) or methylthiomethane is an organosulfur compound with the formula (CH3)2S. Dimethyl sulfide is a flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize, cabbage, beetroot, and seafoods. It is also an indication of bacterial contamination in malt production and brewing. It is a breakdown product of dimethylsulfoniopropionate (DMSP), and is also produced by the bacterial metabolism of methanethiol. Occurrence and production DMS originates primarily from DMSP, a major secondary metabolite in some marine algae. DMS is the most abundant biological sulfur compound emitted to the atmosphere. Emission occurs over the oceans by phytoplankton. DMS is also produced naturally by bacterial transformation of dimethyl sulfoxide (DMSO) waste that is disposed of into sewers, where it can cause environmental odor problems. DMS is oxidized in the marine atmos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Permissible Exposure Limit
The permissible exposure limit (PEL or OSHA PEL) is a legal limit in the United States for exposure of an employee to a chemical substance or physical agent such as high level noise. Permissible exposure limits are established by the Occupational Safety and Health Administration (OSHA). Most of OSHA's PELs were issued shortly after adoption of the Occupational Safety and Health (OSH) Act in 1970. For chemicals, the chemical regulation is usually expressed in parts per million (ppm), or sometimes in milligrams per cubic meter (mg/m3). Units of measure for physical agents such as noise are specific to the agent. A PEL is usually given as a time-weighted average (TWA), although some are short-term exposure limits (STEL) or ceiling limits. A TWA is the average exposure over a specified period, usually a nominal eight hours. This means that, for limited periods, a worker may be exposed to concentration excursions higher than the PEL, so long as the TWA is not exceeded and any applicab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mo(t-BuS)4
Mo or MO may refer to: Arts and entertainment Fictional characters * Mo, a girl in the ''Horrible Histories'' TV series * Mo, also known as Mortimer, in the novel ''Inkheart'' by Cornelia Funke * Mo, in the webcomic '' Jesus and Mo'' * Mo, the main character in the ''Mo's Mischief'' children's book series * Mo, an ophthalmosaurus from ''The Land Before Time'' franchise * MO (Maintenance Operator), a robot in the Filmation series '' Young Sentinels'' * Mo, a main character in ''Zoey's Extraordinary Playlist'' * M-O (Microbe Obliterator), a robot in film ''WALL-E'' * Mo the clown, a character played by Roy Rene, 20th-century Australian stage comedian * Mo Effanga, in the BBC medical drama series ''Holby City'' * Mo Harris, in the BBC soap opera ''EastEnders'' * Little Mo Mitchell, in the BBC soap opera ''EastEnders'' Films * "Mo" (魔 demon), original title of '' The Boxer's Omen'', a 1983 Hong Kong film * ''Mo'' (2010 film), a television movie about British politician Mo Mowla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |