|
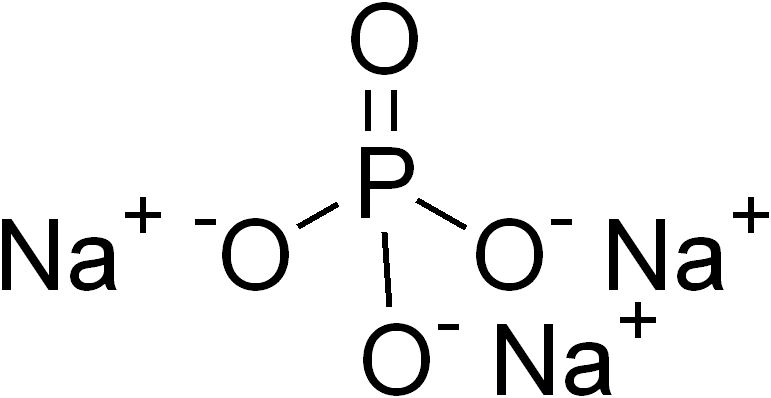
Ternary Compound
In inorganic chemistry and materials chemistry, a ternary compound or ternary phase is a chemical compound containing three different elements. While some ternary compounds are molecular, ''e.g.'' chloroform (), more typically ternary phases refer to extended solids. The perovskites are a famous example. Binary phases, with only two elements, have lower degrees of complexity than ternary phases. With four elements, quaternary phases are more complex. The number of isomers of a ternary compound provide a distinction between inorganic and organic chemistry: "In inorganic chemistry one or, at most, only a few compounds composed of any two or three elements were known, whereas in organic chemistry the situation was very different." Ternary crystalline compounds An example is sodium phosphate, . The sodium ion has a charge of 1+ and the phosphate ion has a charge of 3–. Therefore, three sodium ions are needed to balance the charge of one phosphate ion. Another example of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Chemistry
Inorganic chemistry deals with chemical synthesis, synthesis and behavior of inorganic compound, inorganic and organometallic chemistry, organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, pharmaceutical drug, medications, fuels, and agriculture. Occurrence Many inorganic compounds are found in nature as minerals. Soil may contain iron sulfide as pyrite or calcium sulfate as gypsum. Inorganic compounds are also found multitasking as biomolecules: as electrolytes (sodium chloride), in energy storage (Adenosine triphosphate, ATP) or in construction (the polyphosphate backbone in DNA). Bonding Inorganic compounds exhibit a range ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
III-V Semiconductor
Semiconductor materials are nominally small band gap insulators. The defining property of a semiconductor material is that it can be compromised by doping it with impurities that alter its electronic properties in a controllable way. Because of their application in the computer and photovoltaic industry—in devices such as transistors, lasers, and solar cells—the search for new semiconductor materials and the improvement of existing materials is an important field of study in materials science. Most commonly used semiconductor materials are crystalline inorganic solids. These materials are classified according to the periodic table groups of their constituent atoms. Different semiconductor materials differ in their properties. Thus, in comparison with silicon, compound semiconductors have both advantages and disadvantages. For example, gallium arsenide (GaAs) has six times higher electron mobility than silicon, which allows faster operation; wider band gap, which allows o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semiconductors
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping levels are present in the same crystal, they form a semiconductor junction. The behavior of charge carriers, which include electrons, ions, and electron holes, at these junctions is the basis of diodes, transistors, and most modern electronics. Some examples of semiconductors are silicon, germanium, gallium arsenide, and elements near the so-called "metalloid staircase" on the periodic table. After silicon, gallium arsenide is the second-most common semiconductor and is used in laser diodes, solar cells, microwave-frequency integrated circuits, and others. Silicon is a critical element for fabricating most electronic circuits. Semiconductor devices can display a range of different useful properties, such as passing current more easily in one ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corundum
Corundum is a crystalline form of aluminium oxide () typically containing traces of iron, titanium, vanadium, and chromium. It is a rock (geology), rock-forming mineral. It is a naturally transparency and translucency, transparent material, but can have different colors depending on the presence of transition metal impurities in its crystalline structure. Corundum has two primary gemstone, gem varieties: ruby and sapphire. Rubies are red due to the presence of chromium, and sapphires exhibit a range of colors depending on what transition metal is present. A rare type of sapphire, Sapphire#Padparadscha, padparadscha sapphire, is pink-orange. The name "corundum" is derived from the Tamil language, Tamil-Dravidian languages, Dravidian word ''kurundam'' (ruby-sapphire) (appearing in Sanskrit as ''kuruvinda''). Because of corundum's hardness (pure corundum is defined to have 9.0 on the Mohs scale), it can scratch almost all other minerals. Emery (rock), Emery, a variety of corundum w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyroxene
The pyroxenes (commonly abbreviated Px) are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. Pyroxenes have the general formula , where X represents ions of calcium (Ca), sodium (Na), iron (Fe(II)) or magnesium (Mg) and more rarely zinc, manganese or lithium, and Y represents ions of smaller size, such as chromium (Cr), aluminium (Al), magnesium (Mg), cobalt (Co), manganese (Mn), scandium (Sc), titanium (Ti), vanadium (V) or even iron (Fe(II) or Fe(III)). Although aluminium substitutes extensively for silicon in silicates such as feldspars and amphiboles, the substitution occurs only to a limited extent in most pyroxenes. They share a common structure consisting of single chains of silica tetrahedra. Pyroxenes that crystallize in the monoclinic system are known as clinopyroxenes and those that crystallize in the orthorhombic system are known as orthopyroxenes. The name ''pyroxene'' is derived from the Ancient Greek w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perovskite (structure)
A perovskite is a crystalline material of formula ABX3 with a crystal structure similar to that of the mineral perovskite, this latter consisting of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). In addition to being one of the most abundant structural families, perovskites have wide-ranging properties and applications. Structure Perovskite structures are adopted by many compounds that have the chemical formula ABX3. 'A' and 'B' are positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Derivative (chemistry)
In chemistry, a derivative is a compound that is derived from a similar compound by a chemical reaction. In the past, derivative also meant a compound that ''can be imagined to'' arise from another compound, if one atom or group of atoms is replaced with another atom or group of atoms, but modern chemical language now uses the term structural analog for this meaning, thus eliminating ambiguity. The term "structural analogue" is common in organic chemistry. In biochemistry, the word is used for compounds that at least theoretically can be formed from the precursor compound. Chemical derivatives may be used to facilitate analysis. For example, melting point (MP) analysis can assist in identification of many organic compounds. A crystalline derivative may be prepared, such as a semicarbazone or 2,4-dinitrophenylhydrazone (derived from aldehydes or ketones), as a simple way of verifying the identity of the original compound, assuming that a table of derivative MP values is avai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored. Silicon dioxide is a common fundamental constituent of glass. Structure In the majority of silicon dioxides, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barite
Baryte, barite or barytes ( or ) is a mineral consisting of barium sulfate (Ba S O4). Baryte is generally white or colorless, and is the main source of the element barium. The ''baryte group'' consists of baryte, celestine (strontium sulfate), anglesite (lead sulfate), and anhydrite (calcium sulfate). Baryte and celestine form a solid solution . Names and history The radiating form, sometimes referred to as ''Bologna Stone'', attained some notoriety among alchemists for specimens found in the 17th century near Bologna by Vincenzo Casciarolo. These became phosphorescent upon being calcined. Carl Scheele determined that baryte contained a new element in 1774, but could not isolate barium, only barium oxide. Johan Gottlieb Gahn also isolated barium oxide two years later in similar studies. Barium was first isolated by electrolysis of molten barium salts in 1808 by Sir Humphry Davy in England. The American Petroleum Institute specification API 13/ISO 13500, which governs '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scheelite
Scheelite is a calcium tungstate mineral with the chemical formula Ca W O4. It is an important ore of tungsten (wolfram). Scheelite is originally named after Swedish chemist Carl Wilhelm Scheele (1742–1786). Well-formed crystals are sought by collectors and are occasionally fashioned into gemstones when suitably free of flaws. Scheelite has been synthesized using the Czochralski process; the material produced may be used to imitate diamond, as a scintillator, or as a solid-state lasing medium. It was also used in radium paint in the same fashion as was zinc sulphide, and Thomas Edison invented a fluoroscope with a calcium tungstate-coated screen, making the images six times brighter than those with barium platinocyanide; the latter chemical allowed Röntgen to discover X-rays in early November 1895. The semi-precious stone marketed as 'blue scheelite' is actually a rock type consisting mostly of calcite and dolomite, with occasional traces of yellow-orange scheel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenakite
Phenakite or phenacite is a fairly rare nesosilicate mineral consisting of beryllium orthosilicate, Be2 Si O4. Occasionally used as a gemstone, phenakite occurs as isolated crystals, which are rhombohedral with parallel-faced hemihedrism, and are either lenticular or prismatic in habit: the lenticular habit is determined by the development of faces of several obtuse rhombohedra and the absence of prism faces. There is no cleavage, and the fracture is conchoidal. The Mohs hardness is high, being 7.5–8; the specific gravity is 2.96. The crystals are sometimes perfectly colorless and transparent, but more often they are greyish or yellowish and only translucent; occasionally they are pale rose-red. In general appearance the mineral is not unlike quartz, for which indeed it has been mistaken. Its name comes from , meaning "deceiver" due to its close visual similarity to quartz, named by Nils Gustaf Nordenskiöld in 1833. Largest phenakite A large phenakite gemstone has been fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |