|
Streptomyces Cattleya
{{Streptomyces-stub ...
''Streptomyces cattleya'' is a Gram-positive bacterium which makes cephamycin, penicillin and thienamycin. The bacterium expresses a fluorinase enzyme, and the organism has been used to understand the biosynthesis of fluoroacetate and the antibacterial 4-fluoro-L-threonine. The γ-Glu-βes pathway to biosynthesis of non-traditional amino acids β-ethynylserine (βes) and L-propargylglycine (Pra) was first characterized in this species. The genome, which was sequenced in 2011, contains one chromosome with 6,283,062 base pairs and one megaplasmid with 1,809,491 bp, with an overall guanine-cytosine content of 73%. References cattleya ''Cattleya'' () is a genus of orchids from Costa Rica south to Argentina. The genus is abbreviated C in trade journals. Description Epiphytic or terrestrial orchids with cylindrical rhizome from which the fleshy noodle-like roots grow. Pse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gram-positive
In bacteriology, gram-positive bacteria are bacteria that give a positive result in the Gram stain test, which is traditionally used to quickly classify bacteria into two broad categories according to their type of cell wall. Gram-positive bacteria take up the crystal violet stain used in the test, and then appear to be purple-coloured when seen through an optical microscope. This is because the thick peptidoglycan layer in the bacterial cell wall retains the stain after it is washed away from the rest of the sample, in the decolorization stage of the test. Conversely, gram-negative bacteria cannot retain the violet stain after the decolorization step; alcohol used in this stage degrades the outer membrane of gram-negative cells, making the cell wall more porous and incapable of retaining the crystal violet stain. Their peptidoglycan layer is much thinner and sandwiched between an inner cell membrane and a bacterial outer membrane, causing them to take up the counterstain ( s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cephamycin
Cephamycins are a group of β-lactam antibiotics. They are very similar to cephalosporins, and the cephamycins are sometimes classified as cephalosporins. Like cephalosporins, cephamycins are based upon the cephem nucleus. Unlike most cephalosporins, cephamycins are a very efficient antibiotic against anaerobic microbes. Cephamycins were originally produced by '' Streptomyces'', but synthetic ones have been produced as well. Cephamycins possess a methoxy In organic chemistry, a methoxy group is the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula . On a benzene ring, the Hammett equation classifies a methoxy substituent at the ''para'' position as ... group at the 7-alpha position. In addition, cephamycins have been shown to be stable against extended-spectrum beta-lactamase (ESBL) producing organisms, although their use in clinical practice is lacking for this indication. Examples Cephamycins include: * Cefoxitin * Cefote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Penicillin
Penicillins (P, PCN or PEN) are a group of β-lactam antibiotics originally obtained from '' Penicillium'' moulds, principally '' P. chrysogenum'' and '' P. rubens''. Most penicillins in clinical use are synthesised by P. chrysogenum using deep tank fermentation and then purified. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G (intramuscular or intravenous use) and penicillin V (given by mouth). Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are still widely used today for different bacterial infections, though many types of bacteria have developed resistance following extensive use. 10% of the population claims penicillin allergies but because the frequency of positive skin test results decreases by 10% with each year of avoidance, 90% of these patients can tolerate penicillin. Additionally, those wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thienamycin
Thienamycin (also known as thienpenem) is one of the most potent naturally produced antibiotics known thus far, discovered in '' Streptomyces cattleya'' in 1976. Thienamycin has excellent activity against both Gram-positive and Gram-negative bacteria and is resistant to bacterial β-lactamase enzymes. Thienamycin is a zwitterion at pH 7. History In 1976, fermentation broths obtained from the soil bacterium '' Streptomyces cattleya'' were found to be active in a screen for inhibitors of peptidoglycan biosynthesis. Initial attempts to isolate the active compound proved difficult due to its chemical instability. After many attempts and extensive purification, the material was finally isolated in >90% purity, allowing for the structural elucidation of thienamycin in 1979 (Figure 1). Thienamycin was the first among the naturally occurring class of carbapenem antibiotics to be discovered and isolated. Carbapenems are similar in structure to their antibiotic “cousins” the peni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
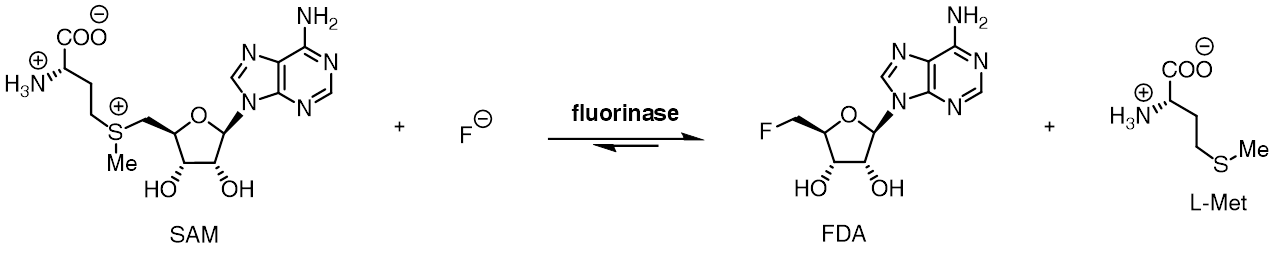
Fluorinase
The fluorinase enzyme (, also known as adenosyl-fluoride synthase) catalyzes the reaction between fluoride ion and the co-factor '' S'' -adenosyl-L-methionine to generate L-methionine and 5'-fluoro-5'-deoxyadenosine, the first committed product of the fluorometabolite biosynthesis pathway. The fluorinase was originally isolated from the soil bacterium '' Streptomyces cattleya'', but homologues have since been identified in a number of other bacterial species, including ''Streptomyces'' sp. MA37, '' Nocardia brasiliensis'' and ''Actinoplanes'' sp. N902-109. This is the only known enzyme capable of catalysing the formation of a carbon-fluorine bond, the strongest single bond in organic chemistry. A homologous chlorinase enzyme, which catalyses the same reaction with chloride rather than fluoride ion, has been isolated from ''Salinospora tropica'', from the biosynthetic pathway of salinosporamide A. Reactivity The fluorinase catalyses an SN2-type nucleophilic substitution at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoroacetate
Fluoroacetate may refer to: * Fluoroacetic acid * Sodium fluoroacetate {{Short pages monitor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-fluoro-L-threonine
4-Fluoro--threonine is an antibacterial produced by '' Streptomyces cattleya''. It is formed by the fluorothreonine transaldolase catalysed transfer of fluoroacetaldehyde Fluoroacetaldehyde is a metabolic precursor of both fluoroacetate and 4-fluorothreonine in ''Streptomyces cattleya''. References {{reflist Organofluorides Aldehydes Fluorine-containing natural products ... onto threonine. References Fluorinated amino acids Fluorohydrins Fluorine-containing natural products {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |