|
Strontium Sulfate
Strontium sulfate (SrSO4) is the sulfate salt of strontium. It is a white crystalline powder and occurs in nature as the mineral celestine. It is poorly soluble in water to the extent of 1 part in 8,800. It is more soluble in dilute HCl and nitric acid and appreciably soluble in alkali chloride solutions (e.g. sodium chloride). Structure Strontium sulfate is a polymeric material, isostructural with barium sulfate. Crystallized strontium sulfate is utilized by a small group of radiolarian protozoa, called the Acantharea, as a main constituent of their skeleton. Applications and chemistry Strontium sulfate is of interest as a naturally occurring precursor to other strontium compounds, which are more useful. In industry it is converted to the carbonate for use as ceramic precursor and the nitrate for use in pyrotechnics. The low aqueous solubility of strontium sulfate can lead to scale formation in processes where these ions meet. For example, it can form on surfaces of equip ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Celestine (mineral)
Celestine (the IMA-accepted name) or celestite is a mineral consisting of strontium sulfate ( Sr S O). The mineral is named for its occasional delicate blue color. Celestine and the carbonate mineral strontianite are the principal sources of the element strontium, commonly used in fireworks and in various metal alloys. Etymology Celestine derives its name from the Latin word ''caelestis'' meaning celestial which in turn is derived from the Latin word ''caelum'' meaning sky, air, weather, atmosphere and heaven. Occurrence Celestine occurs as crystals, and also in compact massive and fibrous forms. It is mostly found in sedimentary rocks, often associated with the minerals gypsum, anhydrite, and halite. On occasion in some localities, it may also be found with sulfur inclusions. The mineral is found worldwide, usually in small quantities. Pale blue crystal specimens are found in Madagascar. White and orange variants also occurred at Yate, Bristol, UK, where it was ext ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as synthetic dyes and medicines (e.g. metronidazole). Nitric acid is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium Compounds
Strontium is a chemical element; it has symbol Sr and atomic number 38. An alkaline earth metal, it is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to air. Strontium has physical and chemical properties similar to those of its two vertical neighbors in the periodic table, calcium and barium. It occurs naturally mainly in the minerals celestine and strontianite, and is mostly mined from these. Both strontium and strontianite are named after Strontian, a village in Scotland near which the mineral was discovered in 1790 by Adair Crawford and William Cruickshank; it was identified as a new element the next year from its crimson-red flame test color. Strontium was first isolated as a metal in 1808 by Humphry Davy using the then newly discovered process of electrolysis. During the 19th century, strontium was mostly used in the production of sugar from sugar beets (see strontian process ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Groundwater
Groundwater is the water present beneath Earth's surface in rock and Pore space in soil, soil pore spaces and in the fractures of stratum, rock formations. About 30 percent of all readily available fresh water in the world is groundwater. A unit of rock or an unconsolidated deposit is called an ''aquifer'' when it can yield a usable quantity of water. The depth at which soil pore spaces or fractures and voids in rock become completely saturated with water is called the ''water table''. Groundwater is Groundwater recharge, recharged from the surface; it may discharge from the surface naturally at spring (hydrosphere), springs and Seep (hydrology), seeps, and can form oasis, oases or wetlands. Groundwater is also often withdrawn for agricultural, municipal, and industrial use by constructing and operating extraction water well, wells. The study of the distribution and movement of groundwater is ''hydrogeology'', also called groundwater hydrology. Typically, groundwater is thought o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oil Well
An oil well is a drillhole boring in Earth that is designed to bring petroleum oil hydrocarbons to the surface. Usually some natural gas is released as associated petroleum gas along with the oil. A well that is designed to produce only gas may be termed a gas well. Wells are created by drilling down into an oil or gas reserve and if necessary equipped with extraction devices such as pumpjacks. Creating the wells can be an expensive process, costing at least hundreds of thousands of dollars, and costing much more when in difficult-to-access locations, e.g., offshore. The process of modern drilling for wells first started in the 19th century but was made more efficient with advances to oil drilling rigs and technology during the 20th century. Wells are frequently sold or exchanged between different oil and gas companies as an asset – in large part because during falls in the price of oil and gas, a well may be unproductive, but if prices rise, even low-production wells may ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fouling
Fouling is the accumulation of unwanted material on solid surfaces. The fouling materials can consist of either living organisms (biofouling, organic) or a non-living substance (inorganic). Fouling is usually distinguished from other surface-growth phenomena in that it occurs on a surface of a component, system, or plant performing a defined and useful function and that the fouling process impedes or interferes with this function. Other terms used in the literature to describe fouling include deposit formation, encrustation, crudding, deposition, scaling, scale formation, slagging, and sludge formation. The last six terms have a more narrow meaning than fouling within the scope of the fouling science and technology, and they also have meanings outside of this scope; therefore, they should be used with caution. Fouling phenomena are common and diverse, ranging from fouling of ship hulls, natural surfaces in the marine environment (fouling community, marine fouling), fouling ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium Nitrate
Strontium nitrate is an inorganic compound composed of the elements strontium, nitrogen and oxygen with the formula Sr( NO3)2. This colorless solid is used as a red colorant and oxidizer in pyrotechnics. Preparation Strontium nitrate is typically generated by the reaction of nitric acid on strontium carbonate. : 2 HNO3 + SrCO3 → Sr(NO3)2 + H2O + CO2 Uses Like many other strontium salts, strontium nitrate is used to produce a rich red flame in fireworks and road flares. The oxidizing properties of this salt are advantageous in such applications.MacMillan, J. Paul; Park, Jai Won; Gerstenberg, Rolf; Wagner, Heinz; Köhler, Karl and Wallbrecht, Peter (2002) "Strontium and Strontium Compounds" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. Strontium nitrate can aid in eliminating and lessening skin irritations. When mixed with glycolic acid, strontium nitrate reduces the sensation of skin irritation significantly better than using glycolic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium Carbonate
Strontium carbonate (SrCO3) is the carbonate salt of strontium that has the appearance of a white or grey powder. It occurs in nature as the mineral strontianite. Chemical properties Strontium carbonate is a white, odorless, tasteless powder. Being a carbonate, it is a weak base and therefore is reactive with acids. It is otherwise stable and safe to work with. It is practically insoluble in water (0.0001 g per 100 ml). The solubility is increased significantly if the water is saturated with carbon dioxide, to 0.1 g per 100 ml. Preparation Other than the natural occurrence as a mineral, strontium carbonate is prepared synthetically in one of two processes, both of which start with naturally occurring celestine, a mineral form of strontium sulfate (SrSO4). In the "black ash" process, celesite is roasted with coke at 1100–1300 °C to form strontium sulfide. The sulfate is reduced, leaving the sulfide: :SrSO4 + 2 C → SrS + 2 CO2 A mixture ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Skeleton
A skeleton is the structural frame that supports the body of most animals. There are several types of skeletons, including the exoskeleton, which is a rigid outer shell that holds up an organism's shape; the endoskeleton, a rigid internal frame to which the organs and soft tissues attach; and the hydroskeleton, a flexible internal structure supported by the hydrostatic pressure of body fluids. Vertebrates are animals with an endoskeleton centered around an axial vertebral column, and their skeletons are typically composed of bones and cartilages. Invertebrates are other animals that lack a vertebral column, and their skeletons vary, including hard-shelled exoskeleton (arthropods and most molluscs), plated internal shells (e.g. cuttlebones in some cephalopods) or rods (e.g. ossicles in echinoderms), hydrostatically supported body cavities (most), and spicules (sponges). Cartilage is a rigid connective tissue that is found in the skeletal systems of vertebrates and invert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
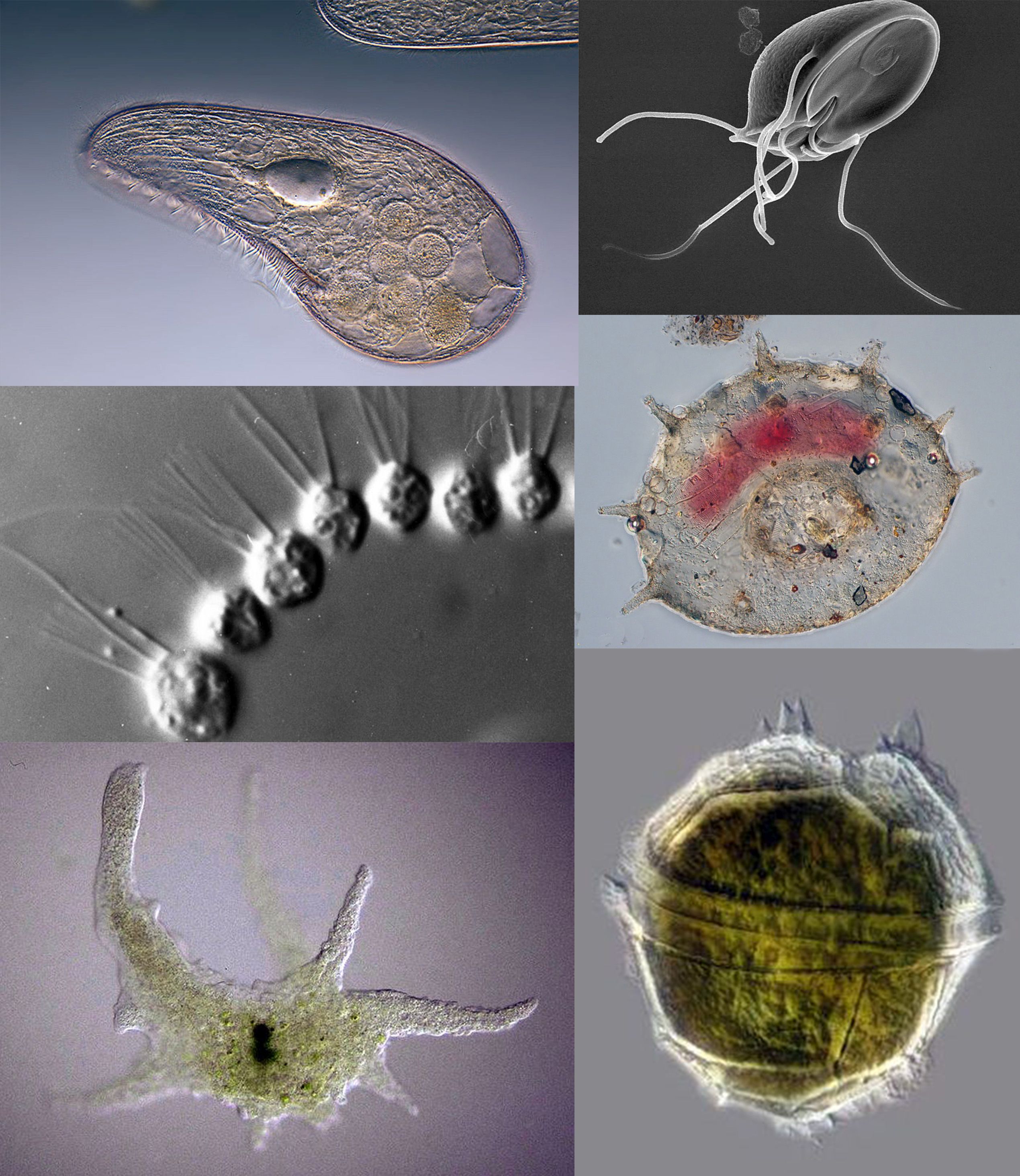
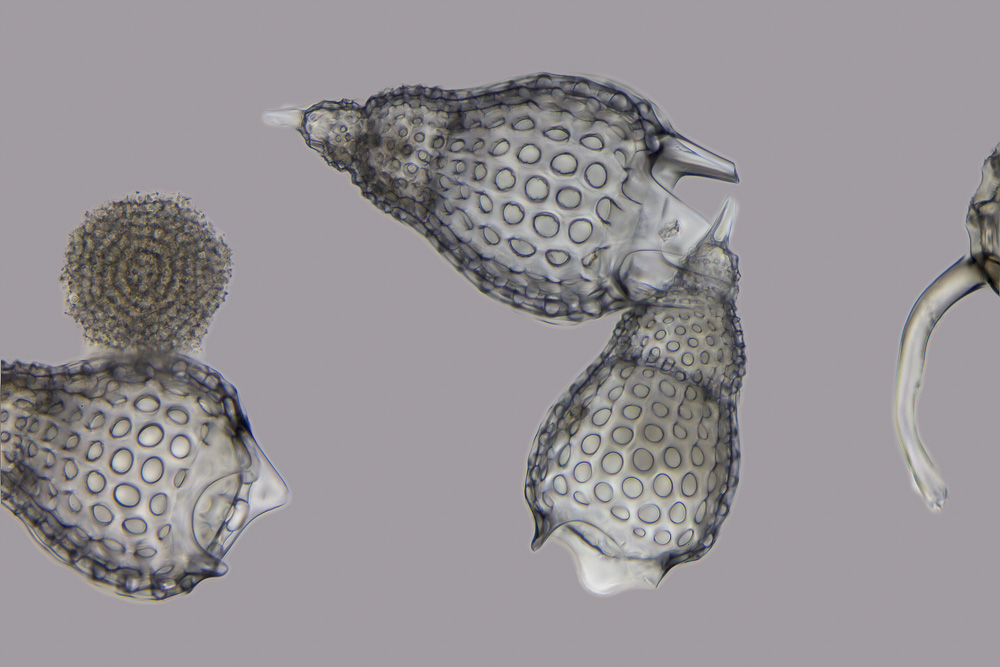
Acantharea
The Acantharia are a group of radiolarian protozoa, distinguished mainly by their strontium sulfate skeletons. Acantharians are heterotrophic marine Plankton, microplankton that range in size from about 200 microns in diameter up to several millimeters. Some acantharians have photosynthetic endosymbionts and hence are considered mixotrophs. Morphology Acantharian skeletons are composed of strontium sulfate, SrSO4, in the form of mineral Celestine (mineral), celestine crystal. Celestine is named for the delicate blue colour of its crystals, and is the heaviest mineral in the ocean. The denseness of their celestite ensures acantharian shells function as Ballast minerals, mineral ballast, resulting in fast sedimentation to bathypelagic, bathypelagic depths. High settling flux, fluxes of acantharian cysts have been observed at times in the Iceland Basin and the Southern Ocean, as much as half of the total gravitational organic carbon flux. Material was copied from this source, whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protozoa
Protozoa (: protozoan or protozoon; alternative plural: protozoans) are a polyphyletic group of single-celled eukaryotes, either free-living or parasitic, that feed on organic matter such as other microorganisms or organic debris. Historically, protozoans were regarded as "one-celled animals". When first introduced by Georg Goldfuss, in 1818, the taxon Protozoa was erected as a class within the Animalia, with the word 'protozoa' meaning "first animals", because they often possess animal-like behaviours, such as motility and predation, and lack a cell wall, as found in plants and many algae. This classification remained widespread in the 19th and early 20th century, and even became elevated to a variety of higher ranks, including phylum, subkingdom, kingdom, and then sometimes included within the paraphyletic Protoctista or Protista. By the 1970s, it became usual to require that all taxa be monophyletic (derived from a common ancestor that would also be regarded as protozo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiolaria
The Radiolaria, also called Radiozoa, are unicellular eukaryotes of diameter 0.1–0.2 mm that produce intricate mineral skeletons, typically with a central capsule dividing the cell into the inner and outer portions of endoplasm and ectoplasm. The elaborate mineral skeleton is usually made of silica. They are found as zooplankton throughout the global ocean. As zooplankton, radiolarians are primarily heterotrophic, but many have photosynthetic endosymbionts and are, therefore, considered mixotrophs. The skeletal remains of some types of radiolarians make up a large part of the cover of the ocean floor as siliceous ooze. Due to their rapid change as species and intricate skeletons, radiolarians represent an important diagnostic fossil found from the Cambrian onwards. Description Radiolarians have many needle-like pseudopods supported by bundles of microtubules, which aid in the radiolarian's buoyancy. The cell nucleus and most other organelles are in the endoplasm, wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |