|
Reflections On The Motive Power Of Fire
''Reflections on the Motive Power of Fire and on Machines Fitted to Develop that Power'' is a book published in 1824 by French physicist Sadi Carnot.full text of 1897 ed. ( Full text of 1897 edition on Wikisource ) The 118-page book's French title was ''Réflexions sur la puissance motrice du feu et sur les machines propres à développer cette puissance''. It is a significant publication in the history of thermodynamics about a generalized theory of heat engines. Overview The book is considered the founding work of thermodynamics. It contains the preliminary outline of the second law of thermodynamics. Carnot stated that motive power is due to the fall of caloric (heat) from a hot to a cold body. The work was unnoticed until 1834 when French mining engineer Émile Clapeyron put it on a graphical footing in his ''Memoir on the Motive Power of Heat''. Through Clapeyron's paper, German physicist Rudolf Clausius learned of Carnot's theory of heat and through a modification of Ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carnot Title Page
Carnot may refer to: People *Carnot Posey (1818–1863), American lawyer and military officer People with the surname *Lazare Carnot (1753-1823), French mathematician and politician of the French Revolution *Louis Carnot (born 2001), French French footballer *Nicolas Léonard Sadi Carnot (1796-1832), French military scientist and physicist; son of Lazare Carnot *Hippolyte Carnot (1801-1888), French politician; son of Lazare Carnot *Marie François Sadi Carnot (1837-1894), French politician; President of France from 1887 to 1894 and son of Hippolyte Carnot *Marie-Adolphe Carnot (1839-1920), French mining engineer and chemist; son of Hippolyte Carnot *Paul Carnot (1869-1957), French physician; son of Marie-Adolphe Carnot *Stéphane Carnot (born 1972), former French footballer Places *Carnot, Central African Republic, a city *Carnot, Wisconsin, United States *Carnot-Moon, Pennsylvania, United States Other uses *Carnot cycle, in thermodynamics *Carnot heat engine, an idealised ther ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theory Of Heat
The history of thermodynamics is a fundamental strand in the history of physics, the history of chemistry, and the history of science in general. Owing to the relevance of thermodynamics in much of science and technology, its history is finely woven with the developments of classical mechanics, quantum mechanics, magnetism, and chemical kinetics, to more distant applied fields such as meteorology, information theory, and biology (physiology), and to technological developments such as the steam engine, internal combustion engine, cryogenics and electricity generation. The development of thermodynamics both drove and was driven by atomic theory. It also, albeit in a subtle manner, motivated new directions in probability and statistics; see, for example, the timeline of thermodynamics. History Contributions from antiquity The ancients viewed heat as that related to fire. In 3000 BC, the ancient Egyptians viewed heat as related to origin mythologies. The ancient Indian philosophy in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Timeline Of Thermodynamics
A chronology, timeline of events in the history of thermodynamics, history of thermodynamics. Before 1800 * 1650 – Otto von Guericke builds the first vacuum pump * 1660 – Robert Boyle experimentally discovers Boyle's Law, relating the pressure and volume of a gas (published 1662) * 1665 – Robert Hooke published his book ''Micrographia'', which contained the statement: "Heat being nothing else but a very brisk and vehement agitation of the parts of a body." * 1667 – J. J. Becher puts forward a theory of combustion involving ''combustible earth'' in his book ''Physica subterranea'' (see Phlogiston theory). * 1676–1689 – Gottfried Leibniz develops the concept of ''vis viva'', a limited version of the conservation of energy * 1679 – Denis Papin designed a steam digester which inspired the development of the piston-and-cylinder steam engine. * 1694–1734 – Georg Ernst Stahl names Becher's combustible earth as phlogiston and develop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
First Law Of Thermodynamics
The first law of thermodynamics is a formulation of the law of conservation of energy, adapted for thermodynamic processes. It distinguishes in principle two forms of energy transfer, heat and thermodynamic work for a system of a constant amount of matter. The law also defines the internal energy of a system, an extensive property for taking account of the balance of energies in the system. The law of conservation of energy states that the total energy of any isolated system, which cannot exchange energy or matter, is constant. Energy can be transformed from one form to another, but can be neither created nor destroyed. The first law for a thermodynamic process is often formulated asThe sign convention (Q is heat supplied ''to'' the system but W is work done ''by'' the system) is that of Rudolf Clausius (Equation IIa on page 384 of Clausius, R. (1850)), and it is followed below. :\Delta U = Q - W, where \Delta U denotes the change in the internal energy of a closed system (f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanical Equivalent Of Heat
In the history of science, the mechanical equivalent of heat states that motion and heat are mutually interchangeable and that in every case, a given amount of work would generate the same amount of heat, provided the work done is totally converted to heat energy. The mechanical equivalent of heat was a concept that had an important part in the development and acceptance of the conservation of energy and the establishment of the science of thermodynamics in the 19th century. History and priority dispute Benjamin Thompson, Count Rumford, had observed the frictional heat generated by boring cannon at the arsenal in Munich, Germany circa 1797. Rumford immersed a cannon barrel in water and arranged for a specially blunted boring tool. He showed that the water could be boiled within roughly two and a half hours and that the supply of frictional heat was seemingly inexhaustible. Based on his experiments, he published "An Experimental Enquiry Concerning the Source of the Heat which is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
James Joule
James Prescott Joule (; 24 December 1818 11 October 1889) was an English physicist, mathematician and brewer, born in Salford, Lancashire. Joule studied the nature of heat, and discovered its relationship to mechanical work (see energy). This led to the law of conservation of energy, which in turn led to the development of the first law of thermodynamics. The SI derived unit of energy, the joule, is named after him. He worked with Lord Kelvin to develop an absolute thermodynamic temperature scale, which came to be called the Kelvin scale. Joule also made observations of magnetostriction, and he found the relationship between the current through a resistor and the heat dissipated, which is also called Joule's first law. His experiments about energy transformations were first published in 1843. Early years James Joule was born in 1818, the son of Benjamin Joule (1784–1858), a wealthy brewer, and his wife, Alice Prescott, on New Bailey Street in Salford. Joule was tu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Efficiency
In thermodynamics, the thermal efficiency (\eta_) is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, steam turbine, steam engine, boiler, furnace, refrigerator, ACs etc. For a heat engine, thermal efficiency is the ratio of the net work output to the heat input; in the case of a heat pump, thermal efficiency (known as the ''coefficient of performance'') is the ratio of net heat output (for heating), or the net heat removed (for cooling) to the energy input (external work). The efficiency of a heat engine is fractional as the output is always less than the input while the COP of a heat pump is more than 1. These values are further restricted by the Carnot theorem. Overview In general, energy conversion efficiency is the ratio between the useful output of a device and the input, in energy terms. For thermal efficiency, the input, Q_, to the device is heat, or the heat-content of a fuel that is consumed. The desir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
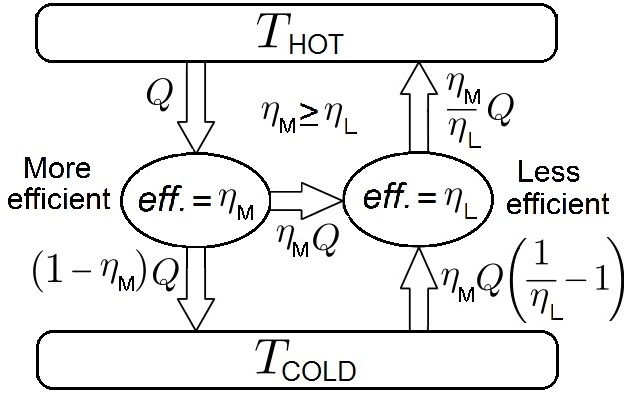
Carnot Theorem (thermodynamics)
In thermodynamics, Carnot's theorem, developed in 1824 by Nicolas Léonard Sadi Carnot, also called Carnot's rule, is a principle that specifies limits on the maximum efficiency that any heat engine can obtain. Carnot's theorem states that all heat engines operating between the same two thermal or heat reservoirs can't have efficiencies greater than a reversible heat engine operating between the same reservoirs. A corollary of this theorem is that every reversible heat engine operating between a pair of heat reservoirs is equally efficient, regardless of the working substance employed or the operation details. Since a Carnot heat engine is also a reversible engine, the efficiency of all the reversible heat engines is determined as the efficiency of the Carnot heat engine that depends solely on the temperatures of its hot and cold reservoirs. The maximum efficiency (i.e., the Carnot heat engine efficiency) of a heat engine operating between cold and hot reservoirs, denoted as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carnot Heat Engine
A Carnot heat engine is a heat engine that operates on the Carnot cycle. The basic model for this engine was developed by Nicolas Léonard Sadi Carnot in 1824. The Carnot engine model was graphically expanded by Benoît Paul Émile Clapeyron in 1834 and mathematically explored by Rudolf Clausius in 1857, work that led to the fundamental thermodynamic concept of entropy. The Carnot engine is the most efficient heat engine which is theoretically possible. The efficiency depends only upon the absolute temperatures of the hot and cold heat reservoirs between which it operates. A heat engine acts by transferring energy from a warm region to a cool region of space and, in the process, converting some of that energy to mechanical work. The cycle may also be reversed. The system may be worked upon by an external force, and in the process, it can transfer thermal energy from a cooler system to a warmer one, thereby acting as a refrigerator or heat pump rather than a heat engine. Every th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carnot Cycle
A Carnot cycle is an ideal thermodynamic cycle proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. By Carnot's theorem, it provides an upper limit on the efficiency of any classical thermodynamic engine during the conversion of heat into work, or conversely, the efficiency of a refrigeration system in creating a temperature difference through the application of work to the system. In a Carnot cycle, a system or engine transfers energy in the form of heat between two thermal reservoirs at temperatures T_H and T_C (referred to as the hot and cold reservoirs, respectively), and a part of this transferred energy is converted to the work done by the system. The cycle is reversible, and there is no generation of entropy. (In other words, entropy is conserved; entropy is only transferred between the thermal reservoirs and the system without gain or loss of it.) When work is applied to the system, heat moves from the cold to hot reser ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
William Thomson, 1st Baron Kelvin
William Thomson, 1st Baron Kelvin, (26 June 182417 December 1907) was a British mathematician, mathematical physicist and engineer born in Belfast. Professor of Natural Philosophy at the University of Glasgow for 53 years, he did important work in the mathematical analysis of electricity and formulation of the first and second laws of thermodynamics, and did much to unify the emerging discipline of physics in its contemporary form. He received the Royal Society's Copley Medal in 1883, was its president 1890–1895, and in 1892 was the first British scientist to be elevated to the House of Lords. Absolute temperatures are stated in units of kelvin in his honour. While the existence of a coldest possible temperature ( absolute zero) was known prior to his work, Kelvin is known for determining its correct value as approximately −273.15 degrees Celsius or −459.67 degrees Fahrenheit. The Joule–Thomson effect is also named in his honour. He worked closely with mathematics ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the microscopic description of nature in statistical physics, and to the principles of information theory. It has found far-ranging applications in chemistry and physics, in biological systems and their relation to life, in cosmology, economics, sociology, weather science, climate change, and information systems including the transmission of information in telecommunication. The thermodynamic concept was referred to by Scottish scientist and engineer William Rankine in 1850 with the names ''thermodynamic function'' and ''heat-potential''. In 1865, German physicist Rudolf Clausius, one of the leading founders of the field of thermodynamics, defined it as the quotient of an infinitesimal amount of hea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |