|
Receptor Antagonist
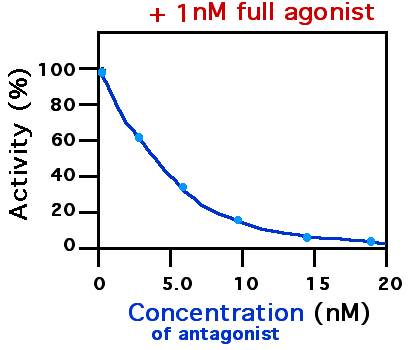
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves " '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, |
Antagonist 2
An antagonist is a character in a story who is presented as the chief foe of the protagonist. Etymology The English word antagonist comes from the Greek language, Greek ἀνταγωνιστής – ''antagonistēs'', "opponent, competitor, villain, enemy, rival," which is derived from ''anti-'' ("against") and ''agonizesthai'' ("to contend for a prize"). Types Heroes and villains The antagonist is commonly positioned against the protagonist and their world order. While most narratives will often portray the protagonist as a hero and the antagonist as a villain, like Harry Potter (Character), Harry Potter and Lord Voldemort in ''Harry Potter'', the antagonist does not always appear as the villain. In some narratives, like Light Yagami and L (Death Note), L in ''Death Note'', the protagonist is a villain and the antagonist is an opposing hero. Antagonists are conventionally presented as making moral choices less savory than those of protagonists. This condition is often used by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand (biochemistry)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association or docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems. In contrast to the definition of lig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histamine H1 Receptor
The H1 receptor is a histamine receptor belonging to the family of rhodopsin-like G-protein-coupled receptors. This receptor is activated by the biogenic amine histamine. It is expressed in smooth muscles, on vascular endothelial cells, in the heart, and in the central nervous system. The H1 receptor is linked to an intracellular G-protein (Gq) that activates phospholipase C and the inositol triphosphate (IP3) signalling pathway. Antihistamines, which act on this receptor, are used as anti-allergy drugs. The crystal structure of the receptor has been determined (shown on the right/below) and used to discover new histamine H1 receptor ligands in structure-based virtual screening studies. Function The expression of NF-κB, the transcription factor that regulates inflammatory processes, is promoted by the constitutive activity of the H1 receptor as well as by agonists that bind at the receptor. H1-antihistamines have been shown to attenuate NF-κB expression and mitigate certain in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vasodilation
Vasodilation is the widening of blood vessels. It results from relaxation of smooth muscle cells within the vessel walls, in particular in the large veins, large arteries, and smaller arterioles. The process is the opposite of vasoconstriction, which is the narrowing of blood vessels. When blood vessels dilate, the flow of blood is increased due to a decrease in vascular resistance and increase in cardiac output. Therefore, dilation of arterial blood vessels (mainly the arterioles) decreases blood pressure. The response may be intrinsic (due to local processes in the surrounding tissue) or extrinsic (due to hormones or the nervous system). In addition, the response may be localized to a specific organ (depending on the metabolic needs of a particular tissue, as during strenuous exercise), or it may be systemic (seen throughout the entire systemic circulation). Endogenous substances and drugs that cause vasodilation are termed vasodilators. Such vasoactivity is necessary for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histamine
Histamine is an organic nitrogenous compound involved in local immune responses, as well as regulating physiological functions in the gut and acting as a neurotransmitter for the brain, spinal cord, and uterus. Since histamine was discovered in 1910, it has been considered a local hormone (autocoid) because it lacks the classic endocrine glands to secrete it; however, in recent years, histamine has been recognized as a central neurotransmitter. Histamine is involved in the inflammatory response and has a central role as a mediator of itching. As part of an immune response to foreign pathogens, histamine is produced by basophils and by mast cells found in nearby connective tissues. Histamine increases the permeability of the capillaries to white blood cells and some proteins, to allow them to engage pathogens in the infected tissues. It consists of an imidazole ring attached to an ethylamine chain; under physiological conditions, the amino group of the side-chain is protonate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physiological Antagonists
Physiological agonism describes the action of a substance which ultimately produces the same effects in the body as another substance—as if they were both agonists at the same receptor—without actually binding to the same receptor. Physiological antagonism describes the behavior of a substance that produces effects counteracting those of another substance (a result similar to that produced by an antagonist blocking the action of an agonist at the same receptor) using a mechanism that does not involve binding to the same receptor. Examples Physiological agonists *Epinephrine induces platelet aggregation, and so does hepatocyte growth factor (HGF). Thus, they are physiological agonists to each other. Physiological antagonists *There are several substances that have antihistaminergic action despite not being ligands for the histamine receptor. For instance, epinephrine raises arterial pressure through vasoconstriction mediated by A1- adrenergic receptor activation, in contrast to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor Theory
Receptor theory is the application of receptor models to explain drug behavior. Pharmacological receptor models preceded accurate knowledge of receptors by many years. John Newport Langley and Paul Ehrlich introduced the concept of a receptor that would mediate drug action at the beginning of the 20th century. Alfred Joseph Clark was the first to quantify drug-induced biological responses (specifically, f-mediated receptor activation). So far, nearly all of the quantitative theoretical modelling of receptor function has centred on ligand-gated ion channels and GPCRs. History The receptor concept In 1901, Langley challenged the dominant hypothesis that drugs act at nerve endings by demonstrating that nicotine acted at sympathetic ganglia even after the degeneration of the severed preganglionic nerve endings. In 1905 he introduced the concept of a receptive substance on the surface of skeletal muscle that mediated the action of a drug. Langley postulated that these receptive substan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allosteric Regulation
In biochemistry, allosteric regulation (or allosteric control) is the regulation of an enzyme by binding an effector molecule at a site other than the enzyme's active site. The site to which the effector binds is termed the ''allosteric site'' or ''regulatory site''. Allosteric sites allow effectors to bind to the protein, often resulting in a conformational change and/or a change in protein dynamics. Effectors that enhance the protein's activity are referred to as ''allosteric activators'', whereas those that decrease the protein's activity are called ''allosteric inhibitors''. Allosteric regulations are a natural example of control loops, such as feedback from downstream products or feedforward from upstream substrates. Long-range allostery is especially important in cell signaling. Allosteric regulation is also particularly important in the cell's ability to adjust enzyme activity. The term ''allostery'' comes from the Ancient Greek ''allos'' (), "other", and ''stereos' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Site
In biochemistry and molecular biology, a binding site is a region on a macromolecule such as a protein that binds to another molecule with specificity. The binding partner of the macromolecule is often referred to as a ligand. Ligands may include other proteins (resulting in a protein-protein interaction), enzyme substrates, second messengers, hormones, or allosteric modulators. The binding event is often, but not always, accompanied by a conformational change that alters the protein's function. Binding to protein binding sites is most often reversible (transient and non-covalent), but can also be covalent reversible or irreversible. Function Binding of a ligand to a binding site on protein often triggers a change in conformation in the protein and results in altered cellular function. Hence binding site on protein are critical parts of signal transduction pathways. Types of ligands include neurotransmitters, toxins, neuropeptides, and steroid hormones. Binding sites in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-covalent Interactions
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. The chemical energy released in the formation of non-covalent interactions is typically on the order of 1–5 kcal/ mol (1000–5000 calories per 6.02 molecules). Non-covalent interactions can be classified into different categories, such as electrostatic, π-effects, van der Waals forces, and hydrophobic effects. Non-covalent interactions are critical in maintaining the three-dimensional structure of large molecules, such as proteins and nucleic acids. In addition, they are also involved in many biological processes in which large molecules bind specifically but transiently to one another (see the properties section of the DNA page). These interactions also heavily influence drug design, crystallinity and design of materials, particularly f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitochondrion
A mitochondrion (; ) is an organelle found in the cells of most Eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'' was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase coined by Philip Siekevitz in a 1957 article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). A large number of unicellular organisms, such as microsporidia, parabasalids and diplomonads, have reduced or transformed their mitochondria into other structures. One eukaryote, ''Monocercomonoides'', is known to have completely lost its mitochondria, and one multicellular organism, '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Receptor
In the field of molecular biology, nuclear receptors are a class of proteins responsible for sensing steroids, thyroid hormones, vitamins, and certain other molecules. These receptors work with other proteins to regulate the expression of specific genes thereby controlling the development, homeostasis, and metabolism of the organism. Nuclear receptors bind directly to DNA regulating the expression of adjacent genes; hence these receptors are classified as transcription factors. The regulation of gene expression by nuclear receptors often occurs in the presence of a ligand—a molecule that affects the receptor's behavior. Ligand binding to a nuclear receptor results in a conformational change activating the receptor. The result is up- or down-regulation of gene expression. A unique property of nuclear receptors that differentiates them from other classes of receptors is their direct control of genomic DNA. Nuclear receptors play key roles in both embryonic development a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |