|
Reactions Of Organocopper Reagents
Reactions of organocopper reagents involve species containing copper-carbon bonds acting as nucleophiles in the presence of organic electrophiles. Organocopper reagents are now commonly used in organic synthesis as mild, selective nucleophiles for substitution and conjugate addition reactions.Lipshutz, B. H.; Sengupta, S. '' Org. React.'' 1992, ''41'', 135. Since the discovery that copper(I) halides catalyze the conjugate addition of Grignard reagents in 1941, organocopper reagents have emerged as weakly basic, nucleophilic reagents for substitution and addition reactions. The constitution of organocopper compounds depends on their method of preparation and the various kinds of organocopper reagents exhibit different reactivity profiles. As a result, the scope of reactions involving organocopper reagents is extremely broad. * Organocopper complexes (RCu) are produced when a copper(I) halide and organolithium are combined. In conjunction with Lewis acidic additives such as boron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Species
Chemical species are a specific form of chemical substance or chemically identical molecular entities that have the same molecular energy level at a specified timescale. These entities are classified through bonding types and relative abundance of isotopes. Types of chemical species can be classified based on the type of molecular entity and can be either an atomic, molecular, ionic or radical species. Classification Generally, a chemical species is defined as a chemical identity that has the same set of molecular energy levels in a defined timescale (i.e. an experiment). These energy levels determine the way the chemical species will interact with others through properties such as bonding or isotopic compositions. The chemical species can be an atom, molecule, ion, or radical, with a specific chemical name and chemical formula. In supramolecular chemistry, chemical species are structures created by forming or breaking bonds between molecules, such as hydrogen bonding, dipole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(I) Chloride
Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear green due to the presence of copper(II) chloride (CuCl2). History Copper(I) chloride was first prepared by Robert Boyle and designated rosin of copper in the mid-seventeenth century from mercury(II) chloride ("Venetian sublimate") and copper metal: :HgCl2 + 2 Cu → 2 CuCl + Hg In 1799, Joseph Proust first differentiated two different chlorides of copper. He prepared CuCl (which he called white muriate of copper) by heating CuCl2 at red heat in the absence of air, causing it to lose half of its combined chlorine followed by removing residual CuCl2 by washing with water. An acidic solution of CuCl was formerly used to analyze carbon monoxide content in gases, for example in Hempel's gas apparatus where the CuCl absorbs the carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins. The International Union of Pure and Applied Chemistry (IUPAC) Preferred IUPAC name, recommends using the name "alkene" only for Open-chain compound, acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for Cyclic compound, cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n'' being a >1 natural number (which is two hydrogens less than the corresponding alkane). When ''n'' is four or more, isomers are possible, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiopure
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities which are mirror images of each other and non-superposable. Enantiomer molecules are like right and left hands: one cannot be superposed onto the other without first being converted to its mirror image. It is solely a relationship of chirality and the permanent three-dimensional relationships among molecules or other chemical structures: no amount of re-orientation of a molecule as a whole or conformational change converts one chemical into its enantiomer. Chemical structures with chirality rotate plane-polarized light. A mixture of equal amounts of each enantiomer, a ''racemic mixture'' or a ''racemate'', does not rotate light. Stereoisomers include both enantiomers and diastereomers. Diastereomers, like enantiomers, share the same molecular formula and are also non-superimposable onto ea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' are methyl), with the formula . Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, ''e.g.'', testosterone, and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered retained IUPAC names, although some introdu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fulvestrant Organic Synthesis Brazier 2010
Fulvestrant, sold under the brand name Faslodex among others, is an antiestrogenic medication used to treat hormone receptor (HR)-positive metastatic breast cancer in postmenopausal women with disease progression as well as HR-positive, HER2-negative advanced breast cancer in combination with abemaciclib or palbociclib in women with disease progression after endocrine therapy. It is given by injection into a muscle. Fulvestrant is a selective estrogen receptor degrader (SERD) and was first-in-class to be approved. It works by binding to the estrogen receptor and destabilizing it, causing the cell's normal protein degradation processes to destroy it. Fulvestrant was approved for medical use in the United States in 2002. Medical uses Breast cancer Fulvestrant is used for the treatment of hormone receptor positive metastatic breast cancer or locally advanced unresectable disease in postmenopausal women; it is given by injection. A 2017 Cochrane review found it is as safe and e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydration Reaction
In chemistry, a dehydration reaction is a chemical reaction that involves the loss of an H2O from the reacting molecule(s) or ion(s). This reaction results in the release of the H2O as water. When the reaction involves the coupling of two molecules into a single molecule it is referred to as a condensation reaction. Dehydration reactions are common processes in the manufacture of chemical compounds as well as naturally occurring within living organisms. The reverse of a dehydration reaction is called a hydration reaction. The reverse of a condensation reaction yielding water is called hydrolysis. Condensation reactions occurring in living organisms Condensation dehydration reactions are fundamental to the existence of life as this type of reaction produces proteins from amino acids, DNA and RNA from nucleotides, fats from fatty acids, and polysaccharides (eg. cellulose, starch, sugar, lactose) from monosaccharides (eg. glucose and fructose). The formation of the pyrophosphat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
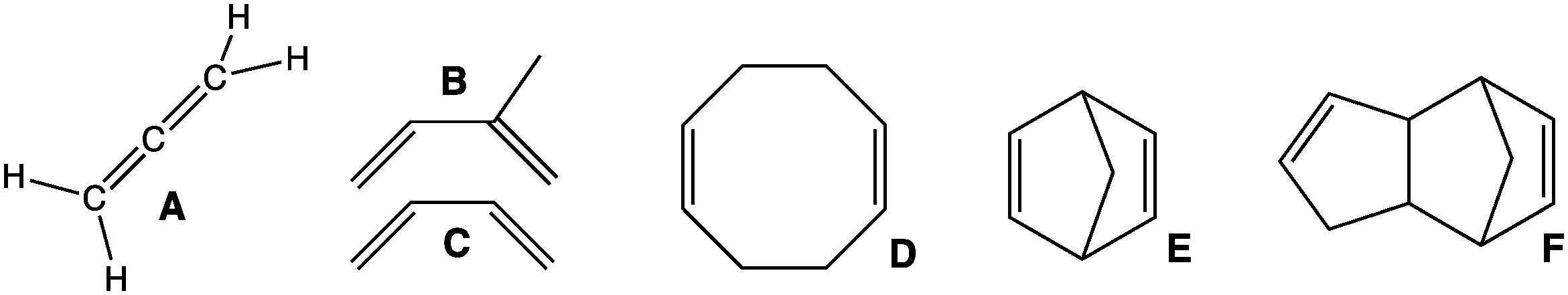
Diene
In organic chemistry, a diene ( ); also diolefin, ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature. As a subunit of more complex molecules, dienes occur in naturally occurring and synthetic chemicals and are used in organic synthesis. Conjugated dienes are widely used as monomers in the polymer industry. Polyunsaturated fats are of interest to nutrition. Classes Dienes can be divided into three classes, depending on the relative location of the double bonds: #Cumulated dienes have the double bonds sharing a common atom. The result is more specifically called an allene. #Conjugated dienes have conjugated double bonds separated by one single bond. Conjugated dienes are more stable than other dienes because of resonance. #Unconjugated dienes have the double bonds separated by two or more single bonds. They are usually less ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |