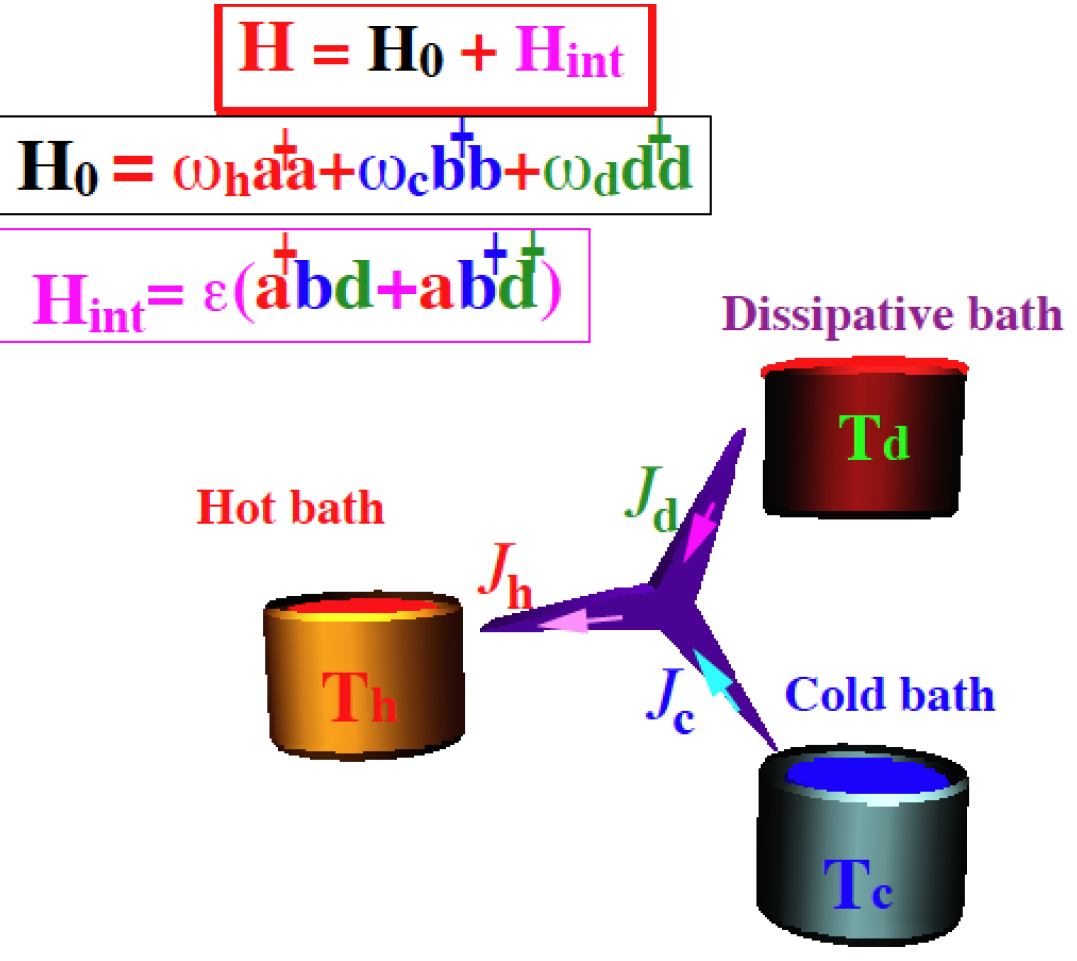
|
Quantum Absorption Refrigerator
A quantum heat engine is a device that generates power from the heat flow between hot and cold reservoirs. The operation mechanism of the engine can be described by the laws of quantum mechanics. The first realization of a quantum heat engine was pointed out by Scovil and Schulz-DuBois in 1959, showing the connection of efficiency of the Carnot engine and the 3-level maser. Quantum refrigerators share the structure of quantum heat engines with the purpose of pumping heat from a cold to a hot bath consuming power first suggested by Geusic, Schulz-DuBois, De Grasse and Scovil. When the power is supplied by a laser the process is termed optical pumping or laser cooling, suggested by Wineland and Hänsch.D. J. Wineland and H. Dehmelt, Bull. Am. Phys. Soc. 20, 637 (1975) Surprisingly heat engines and refrigerators can operate up to the scale of a single particle thus justifying the need for a quantum theory termed quantum thermodynamics. The 3-level amplifier as a quantum heat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Engine
In thermodynamics and engineering, a heat engine is a system that converts heat to mechanical energy, which can then be used to do mechanical work. It does this by bringing a working substance from a higher state temperature to a lower state temperature. A heat source generates thermal energy that brings the working substance to the higher temperature state. The working substance generates work in the working body of the engine while transferring heat to the colder sink until it reaches a lower temperature state. During this process some of the thermal energy is converted into work by exploiting the properties of the working substance. The working substance can be any system with a non-zero heat capacity, but it usually is a gas or liquid. During this process, some heat is normally lost to the surroundings and is not converted to work. Also, some energy is unusable because of friction and drag. In general, an engine is any machine that converts energy to mechanical work. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carnot Cycle
A Carnot cycle is an ideal thermodynamic cycle proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. By Carnot's theorem, it provides an upper limit on the efficiency of any classical thermodynamic engine during the conversion of heat into work, or conversely, the efficiency of a refrigeration system in creating a temperature difference through the application of work to the system. In a Carnot cycle, a system or engine transfers energy in the form of heat between two thermal reservoirs at temperatures T_H and T_C (referred to as the hot and cold reservoirs, respectively), and a part of this transferred energy is converted to the work done by the system. The cycle is reversible, and there is no generation of entropy. (In other words, entropy is conserved; entropy is only transferred between the thermal reservoirs and the system without gain or loss of it.) When work is applied to the system, heat moves from the cold to hot reser ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Demagnetization
In classical electromagnetism, magnetization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material. Movement within this field is described by direction and is either Axial or Diametric. The origin of the magnetic moments responsible for magnetization can be either microscopic electric currents resulting from the motion of electrons in atoms, or the spin of the electrons or the nuclei. Net magnetization results from the response of a material to an external magnetic field. Paramagnetic materials have a weak induced magnetization in a magnetic field, which disappears when the magnetic field is removed. Ferromagnetic and ferrimagnetic materials have strong magnetization in a magnetic field, and can be ''magnetized'' to have magnetization in the absence of an external field, becoming a permanent magnet. Magnetization is not necessarily uniform within a material, but may vary between different points. Magnetiza ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adiabatic Compression
In thermodynamics, an adiabatic process (Greek: ''adiábatos'', "impassable") is a type of thermodynamic process that occurs without transferring heat or mass between the thermodynamic system and its environment. Unlike an isothermal process, an adiabatic process transfers energy to the surroundings only as work.. A translation may be founhere. Also a mostly reliabltranslation is to be foundin As a key concept in thermodynamics, the adiabatic process supports the theory that explains the first law of thermodynamics. Some chemical and physical processes occur too rapidly for energy to enter or leave the system as heat, allowing a convenient "adiabatic approximation".Bailyn, M. (1994), pp. 52–53. For example, the adiabatic flame temperature uses this approximation to calculate the upper limit of flame temperature by assuming combustion loses no heat to its surroundings. In meteorology and oceanography, adiabatic cooling produces condensation of moisture or salinity, oversatur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetization
In classical electromagnetism, magnetization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material. Movement within this field is described by direction and is either Axial or Diametric. The origin of the magnetic moments responsible for magnetization can be either microscopic electric currents resulting from the motion of electrons in atoms, or the spin of the electrons or the nuclei. Net magnetization results from the response of a material to an external magnetic field. Paramagnetic materials have a weak induced magnetization in a magnetic field, which disappears when the magnetic field is removed. Ferromagnetic and ferrimagnetic materials have strong magnetization in a magnetic field, and can be ''magnetized'' to have magnetization in the absence of an external field, becoming a permanent magnet. Magnetization is not necessarily uniform within a material, but may vary between different points. Magneti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isochoric Process
In thermodynamics, an isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant. An isochoric process is exemplified by the heating or the cooling of the contents of a sealed, inelastic container: The thermodynamic process is the addition or removal of heat; the isolation of the contents of the container establishes the closed system; and the inability of the container to deform imposes the constant-volume condition. The isochoric process here should be a quasi-static process. Formalism An isochoric thermodynamic quasi-static process is characterized by constant volume, i.e., . The process does no pressure-volume work, since such work is defined by W = P \Delta V , where is pressure. The sign convention is such that positive work is performed by the system on the environment. If the process is not quasi-static, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stirling Cycle
The Stirling cycle is a thermodynamic cycle that describes the general class of Stirling devices. This includes the original Stirling engine that was invented, developed and patented in 1816 by Robert Stirling with help from his brother, an engineer. The ideal Otto and Diesel cycles are not totally reversible because they involve heat transfer through a finite temperature difference during the irreversible isochoric/isobaric heat-addition and heat-rejection processes. The irreversibility renders the thermal efficiency of these cycles less than that of a Carnot engine operating within the same limits of temperature. Another cycle that features isothermal heat-addition and heat-rejection processes is the Stirling cycle, which is an altered version of the Carnot cycle in which the two isentropic processes featured in the Carnot cycle are replaced by two constant-volume regeneration processes. The cycle is reversible, meaning that if supplied with mechanical power, it can funct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Cycle
A thermodynamic cycle consists of a linked sequence of thermodynamic processes that involve transfer of heat and work into and out of the system, while varying pressure, temperature, and other state variables within the system, and that eventually returns the system to its initial state. In the process of passing through a cycle, the working fluid (system) may convert heat from a warm source into useful work, and dispose of the remaining heat to a cold sink, thereby acting as a heat engine. Conversely, the cycle may be reversed and use work to move heat from a cold source and transfer it to a warm sink thereby acting as a heat pump. If at every point in the cycle the system is in thermodynamic equilibrium, the cycle is reversible. Whether carried out reversible or irreversibly, the net entropy change of the system is zero, as entropy is a state function. During a closed cycle, the system returns to its original thermodynamic state of temperature and pressure. Process quanti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Otto Cycle
An Otto cycle is an idealized thermodynamic cycle that describes the functioning of a typical spark ignition piston engine. It is the thermodynamic cycle most commonly found in automobile engines. The Otto cycle is a description of what happens to a gas as it is subjected to changes of pressure, temperature, volume, addition of heat, and removal of heat. The gas that is subjected to those changes is called the system. The system, in this case, is defined to be the fluid (gas) within the cylinder. By describing the changes that take place within the system, it will also describe in inverse, the system's effect on the environment. In the case of the Otto cycle, the effect will be to produce enough net work from the system so as to propel an automobile and its occupants in the environment. The Otto cycle is constructed from: :Top and bottom of the loop: a pair of quasi-parallel and isentropic processes (frictionless, adiabatic reversible). :Left and right sides of the loop: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lasers
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word "laser" is an acronym for "light amplification by stimulated emission of radiation". The first laser was built in 1960 by Theodore H. Maiman at Hughes Research Laboratories, based on theoretical work by Charles Hard Townes and Arthur Leonard Schawlow. A laser differs from other sources of light in that it emits light which is coherence (physics), ''coherent''. Spatial coherence allows a laser to be focused to a tight spot, enabling applications such as laser cutting and Photolithography#Light sources, lithography. Spatial coherence also allows a laser beam to stay narrow over great distances (collimated light, collimation), enabling applications such as laser pointers and lidar (light detection and ranging). Lasers can also have high temporal coherence, which allows them to emit light with a very narrow frequency spectrum, spectr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoelectric
The thermoelectric effect is the direct conversion of temperature differences to electric voltage and vice versa via a thermocouple. A thermoelectric device creates a voltage when there is a different temperature on each side. Conversely, when a voltage is applied to it, heat is transferred from one side to the other, creating a temperature difference. At the atomic scale, an applied temperature gradient causes charge carriers in the material to diffuse from the hot side to the cold side. This effect can be used to generate electricity, measure temperature or change the temperature of objects. Because the direction of heating and cooling is affected by the applied voltage, thermoelectric devices can be used as temperature controllers. The term "thermoelectric effect" encompasses three separately identified effects: the Seebeck effect, Peltier effect, and Thomson effect. The Seebeck and Peltier effects are different manifestations of the same physical process; textbooks may r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |