|
Protein Fold Class
In molecular biology, protein fold classes are broad categories of protein tertiary structure topology. They describe groups of proteins that share similar amino acid and secondary structure proportions. Each class contains multiple, independent protein superfamilies (i.e. are not necessarily evolutionarily related to one another). Generally recognised classes Four large classes of protein that are generally agreed upon by the two main structure classification databases (SCOP and CATH). all-α All-α proteins are a class of structural domains in which the secondary structure is composed entirely of α-helices, with the possible exception of a few isolated β-sheets on the periphery. Common examples include the bromodomain, the globin fold and the homeodomain fold. all-β All-β proteins are a class of structural domains in which the secondary structure is composed entirely of β-sheets, with the possible exception of a few isolated α-helices on the periphery. C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
A Summary Of Functional Annotation Of The Most Ancestral Translation Protein Folds
A, or a, is the first letter and the first vowel of the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''a'' (pronounced ), plural ''aes''. It is similar in shape to the Ancient Greek letter alpha, from which it derives. The uppercase version consists of the two slanting sides of a triangle, crossed in the middle by a horizontal bar. The lowercase version can be written in two forms: the double-storey a and single-storey ɑ. The latter is commonly used in handwriting and fonts based on it, especially fonts intended to be read by children, and is also found in italic type. In English grammar, " a", and its variant " an", are indefinite articles. History The earliest certain ancestor of "A" is aleph (also written 'aleph), the first letter of the Phoenician alphabet, which consisted entirely of consonants (for that reason, it is also called an abjad to distinguish it f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-propeller Domain
In structural biology, a beta-propeller (β-propeller) is a type of all-β protein architecture characterized by 4 to 8 highly symmetrical blade-shaped beta sheets arranged toroidally around a central axis. Together the beta-sheets form a funnel-like active site. Structure Each beta-sheet typically has four anti-parallel β-strands arranged in the beta-zigzag motif. The strands are twisted so that the first and fourth strands are almost perpendicular to each other. There are five classes of beta-propellers, each arrangement being a highly symmetrical structure with 4–8 beta sheets, all of which generally form a central tunnel that yields pseudo-symmetric axes. While, the protein's official active site for ligand-binding is formed at one end of the central tunnel by loops between individual beta-strands, protein-protein interactions can occur at multiple areas around the domain. Depending on the packing and tilt of the beta-sheets and beta-strands, the beta-propeller may h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scleroprotein
In molecular biology, fibrous proteins or scleroproteins are one of the three main classifications of protein structure (alongside globular and membrane proteins). Fibrous proteins are made up of elongated or fibrous polypeptide chains which form filamentous and sheet-like structures. These kind of protein can be distinguished from globular protein by its low solubility in water. Such proteins serve protective and structural roles by forming connective tissue, tendons, bone matrices, and muscle fiber. Fibrous proteins consist of many superfamilies including keratin, collagen, elastin, and fibrin. Collagen is the most abundant of these proteins which exists in vertebrate connective tissue including tendon, cartilage, and bone. Biomolecular structure A fibrous protein forms long protein filaments, which are shaped like rods or wires. Fibrous proteins are structural or storage proteins that are typically inert and water- insoluble. A fibrous protein occurs as an aggreg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Globular Proteins
In biochemistry, globular proteins or spheroproteins are spherical ("globe-like") proteins and are one of the common protein types (the others being fibrous, disordered and membrane proteins). Globular proteins are somewhat water-soluble (forming colloids in water), unlike the fibrous or membrane proteins. There are multiple fold classes of globular proteins, since there are many different architectures that can fold into a roughly spherical shape. The term globin can refer more specifically to proteins including the globin fold. Globular structure and solubility The term globular protein is quite old (dating probably from the 19th century) and is now somewhat archaic given the hundreds of thousands of proteins and more elegant and descriptive structural motif vocabulary. The globular nature of these proteins can be determined without the means of modern techniques, but only by using ultracentrifuges or dynamic light scattering techniques. The spherical structure is induce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biological Membranes
A biological membrane, biomembrane or cell membrane is a selectively permeable membrane that separates the interior of a cell from the external environment or creates intracellular compartments by serving as a boundary between one part of the cell and another. Biological membranes, in the form of eukaryotic cell membranes, consist of a phospholipid bilayer with embedded, integral and peripheral proteins used in communication and transportation of chemicals and ions. The bulk of lipids in a cell membrane provides a fluid matrix for proteins to rotate and laterally diffuse for physiological functioning. Proteins are adapted to high membrane fluidity environment of the lipid bilayer with the presence of an annular lipid shell, consisting of lipid molecules bound tightly to the surface of integral membrane proteins. The cell membranes are different from the isolating tissues formed by layers of cells, such as mucous membranes, basement membranes, and serous membranes. Compositi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
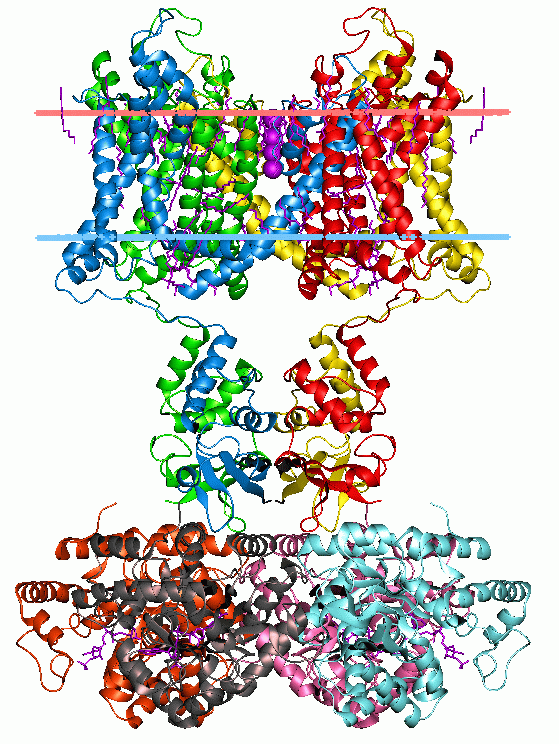
Membrane Protein
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane (transmembrane) or associate with one or the other side of a membrane ( integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane. Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs. Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct conformation of the protein in isolation from its native environment. Function Membrane proteins perform a variety of functions vital to the sur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribonuclease Inhibitor
Ribonuclease inhibitor (RI) is a large (~450 residues, ~49 kDa), acidic (pI ~4.7), leucine-rich repeat protein that forms extremely tight complexes with certain ribonucleases. It is a major cellular protein, comprising ~0.1% of all cellular protein by weight, and appears to play an important role in regulating the lifetime of RNA. RI has a surprisingly high cysteine content (~6.5%, cf. 1.7% in typical proteins) and is sensitive to oxidation. RI is also rich in leucine (21.5%, compared to 9% in typical proteins) and commensurately lower in other hydrophobic residues, esp. valine, isoleucine, methionine, tyrosine, and phenylalanine. Structure RI is the classic leucine-rich repeat protein, consisting of alternating α-helices and β-strands along its backbone. These secondary structure elements wrap around in a curved, right-handed solenoid that resembles a horseshoe. The parallel β-strands and α-helices form the inner and outer wall of the horseshoe, respective ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TIM Barrel
The TIM barrel (triose-phosphate isomerase), also known as an alpha/beta barrel, is a conserved protein fold consisting of eight alpha helices (α-helices) and eight parallel beta strands (β-strands) that alternate along the peptide backbone. The structure is named after triose-phosphate isomerase, a conserved metabolic enzyme. TIM barrels are ubiquitous, with approximately 10% of all enzymes adopting this fold. Further, five of seven enzyme commission (EC) enzyme classes include TIM barrel proteins. The TIM barrel fold is evolutionarily ancient, with many of its members possessing little similarity today, instead falling within the ''twilight zone'' of sequence similarity. The inner beta barrel (β-barrel) is in many cases stabilized by intricate salt-bridge networks. Loops at the C-terminal ends of the β-barrel are responsible for catalytic activity while N-terminal end loops are important for the stability of the TIM-barrels. Structural inserts ranging from extend ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavodoxin Fold
300px, Ribbon diagram of CheY (a regulator of the chemotactic response in bacteria, PDB accession code 3CHY), which adopts the flavodoxin fold. Ribbon is colored from blue (N-terminus) to red (C-terminus). The flavodoxin fold is a common α/β protein fold, second only to the TIM barrel fold. It has three layers, with two alpha helix, α-helical layers sandwiching a 5-stranded parallel β-sheet. The order of strands within the sheet is 2-1-3-4-5. This motif is present for example in lactate dehydrogenase () or phosphoglycerate kinase (). External links SCOP list of proteins adopting the flavodoxin foldmirror References {{Protein tertiary structure Protein folds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SH2 Domain
The SH2 (Src Homology 2) domain is a structurally conserved protein domain contained within the Src oncoprotein and in many other intracellular signal-transducing proteins. SH2 domains allow proteins containing those domains to dock to phosphorylated tyrosine residues on other proteins. SH2 domains are commonly found in adaptor proteins that aid in the signal transduction of receptor tyrosine kinase pathways. Background SH2 is conserved by signalization of protein tyrosine kinase, which are binding on phosphotyrosine (pTyr). In the human proteome the class of pTyr-selective recognition domains is represented by SH2 domains. The N-terminal SH2 domains of cytoplasmic tyrosine kinase was at the beginning of evolution evolved with the occurrence of tyrosine phosphorylation. At the beginning it was supposed that, these domains serve as a substrate for their target kinase. Protein-protein interactions play a major role in cellular growth and development. Modular domains, which are t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribonuclease A
Pancreatic ribonuclease family (, ''RNase'', ''RNase I'', ''RNase A'', ''pancreatic RNase'', ''ribonuclease I'', ''endoribonuclease I'', ''ribonucleic phosphatase'', ''alkaline ribonuclease'', ''ribonuclease'', ''gene S glycoproteins'', ''Ceratitis capitata alkaline ribonuclease'', ''SLSG glycoproteins'', ''gene S locus-specific glycoproteins'', ''S-genotype-assocd. glycoproteins'', ''ribonucleate 3'-pyrimidino-oligonucleotidohydrolase'') is a superfamily of pyrimidine-specific endonucleases found in high quantity in the pancreas of certain mammals and of some reptiles. Specifically, the enzymes are involved in endonucleolytic cleavage of 3'-phosphomono nucleotides and 3'-phosphooligonucleotides ending in C-P or U-P with 2',3'-cyclic phosphate intermediates. Ribonuclease can unwind the RNA helix by complexing with single-stranded RNA; the complex arises by an extended multi-site cation-anion interaction between lysine and arginine residues of the enzyme and phosphate groups of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferredoxin Fold
In protein structure, a ferredoxin fold is a common α+β protein fold with a signature βαββαβ secondary structure along its backbone. Structurally, the ferredoxin fold can be regarded as a long, symmetric hairpin that is wrapped once around, so that its two terminal β-strands hydrogen-bond to the central two β-strands, forming a four-stranded, antiparallel β-sheet covered on one side by two α-helices. External links SCOP list of proteins with a ferredoxin-like fold References Protein folds {{protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |