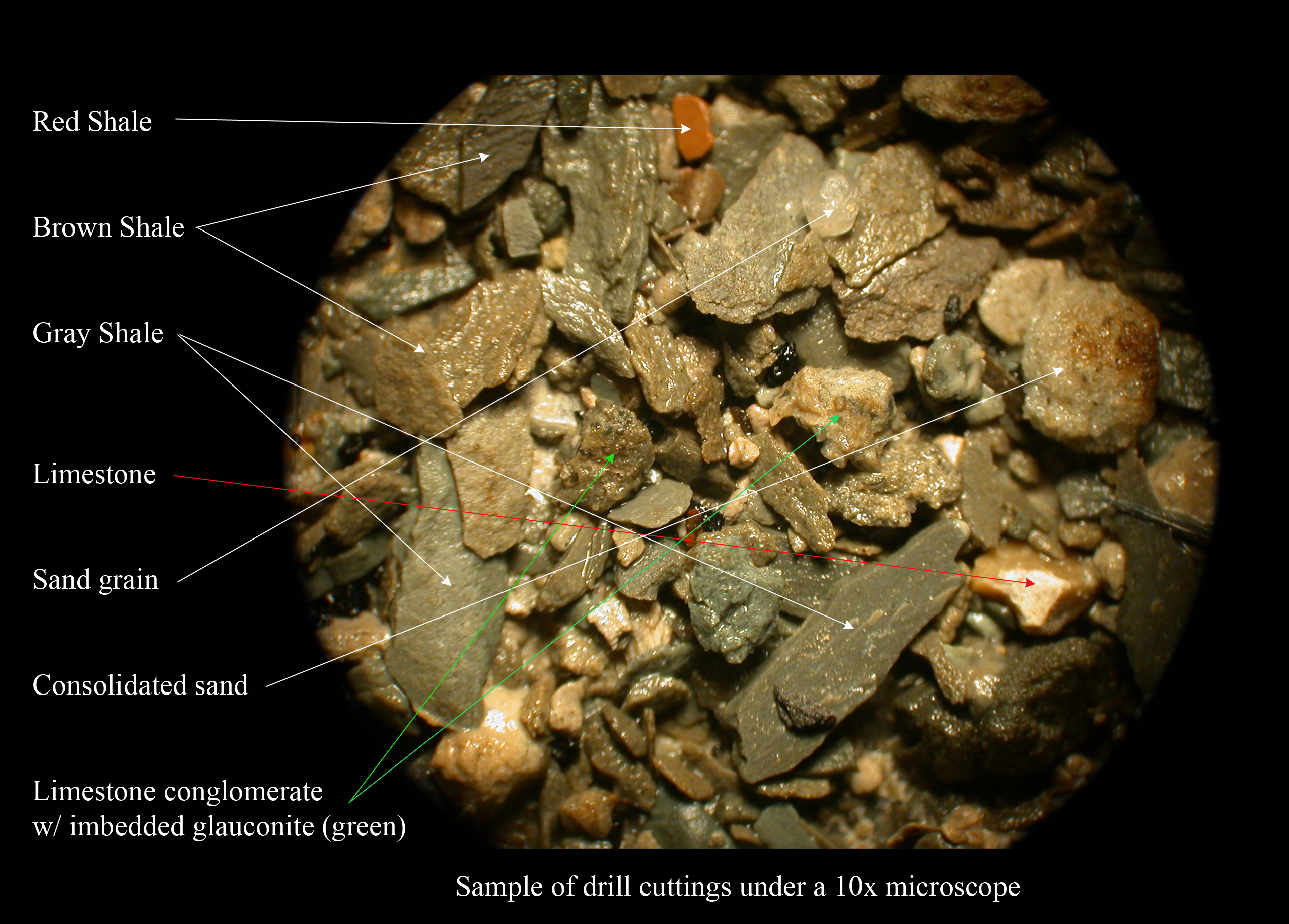
|
Oil-based Mud
Oil-based mud is a drilling fluid used in drilling engineering. It is composed of oil as the continuous phase and water as the dispersed phase in conjunction with emulsifiers, wetting agents and gellants. The oil base can be diesel, kerosene, fuel oil, selected crude oil or mineral oil. The requirements are a gravity of 36–37 API, a flash point of , fire point of and an aniline point of . Emulsifiers are important to oil-based mud due to the likelihood of contamination. The water phase of oil-based mud can be freshwater, or a solution of sodium or calcium chloride. The external phase is oil and does not allow the water to contact the formation. The shales don't become water wet. Poor stability of the emulsion results in the two layers separating into two distinct layers. The advantages are: # high drilling rates # lowered drill pipe torque and drag, # less bit balling and # reduction in differential sticking. Oil-based muds are expensive, but are worth the cost w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drilling Fluid
In geotechnical engineering, drilling fluid, also called drilling mud, is used to aid the drilling of boreholes into the earth. Often used while drilling oil and natural gas wells and on exploration drilling rigs, drilling fluids are also used for much simpler boreholes, such as water wells. One of the functions of drilling mud is to carry cuttings out of the hole. The three main categories of drilling fluids are: water-based muds (WBs), which can be dispersed and non-dispersed; non-aqueous muds, usually called oil-based muds (OBs); and gaseous drilling fluid, in which a wide range of gases can be used. Along with their formatives, these are used along with appropriate polymer and clay additives for drilling various oil and gas formations. The main functions of drilling fluids include providing hydrostatic pressure to prevent formation fluids from entering into the well bore, keeping the drill bit cool and clean during drilling, carrying out drill cuttings, and susp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fire Point
The fire point of a fuel is the lowest temperature at which the vapour of that fuel will continue to burn for at least five seconds after ignition by an open flame of standard dimension. At the flash point, a lower temperature, a substance will ignite briefly, but vapor might not be produced at a rate to sustain the fire. Most tables of material properties will only list material flash points. In general the fire point can be assumed to be about 10 °C higher than the flash point, although this is no substitute for testing if the fire point is safety critical. Testing of the fire point is done by open cup apparatus. ASTM.org See also * |
Dehydrate
In physiology, dehydration is a lack of total body water, with an accompanying disruption of metabolic processes. It occurs when free water loss exceeds free water intake, usually due to exercise, disease, or high environmental temperature. Mild dehydration can also be caused by immersion diuresis, which may increase risk of decompression sickness in divers. Most people can tolerate a 3-4% decrease in total body water without difficulty or adverse health effects. A 5-8% decrease can cause fatigue and dizziness. Loss of over ten percent of total body water can cause physical and mental deterioration, accompanied by severe thirst. Death occurs at a loss of between fifteen and twenty-five percent of the body water.Ashcroft F, Life Without Water in Life at the Extremes. Berkeley and Los Angeles, 2000, 134-138. Mild dehydration is characterized by thirst and general discomfort and is usually resolved with oral rehydration. Dehydration can cause hypernatremia (high levels of sodiu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Smectite
A smectite (from ancient Greek ''σμηκτός'' smektos 'lubricated'; ''σμηκτρίς'' smektris 'walker's earth', 'fuller's earth'; rubbing earth; earth that has the property of cleaning) is a mineral mixtures of various swelling sheet silicates (phyllosilicates), which have a three-layer 2:1 (TOT) structure and belong to the clay minerals. Smectites mainly consist of montmorillonite, but can often contain secondary minerals such as quartz and calcite. Terminology In clay mineralogy, smectite is synonym of montmorillonite (also the name of a pure clay mineral phase) to indicate a class of swelling clays. The term smectite is commonly used in Europe and in the UK while the term montmorillonite is preferred in North America, but both terms are equivalent and can be used interchangeably. For industrial and commercial applications, the term bentonite is mostly used in place of smectite or montmorillonite. Mineralogical structure The 2:1 layer (TOT) structure consists of tw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Differential Sticking
Differential sticking is a problem that occurs when drilling a well with a greater well bore pressure than formation pressure, as is usually the case. The drill pipe is pressed against the wellbore wall so that part of its circumference will see only reservoir pressure, while the rest will continue to be pushed by wellbore pressure. As a result, the pipe becomes stuck to the wall, and can require millions of pounds of force to remove, which may prove impossible. In many cases the drilling fluid (mud) weight is reduced, thus relieving the pressure difference and releasing the stuck pipe string. Should this option be unavailable, as in sour gas Sour gas is natural gas or any other gas containing significant amounts of hydrogen sulfide (H2S). Natural gas is usually considered sour if there are more than 5.7 milligrams of H2S per cubic meter of natural gas, which is equivalent to approxim ... wells, a specialty fishing company is called to retrieve the stuck pipe or 'fish'. Many op ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Torque
In physics and mechanics, torque is the rotational equivalent of linear force. It is also referred to as the moment of force (also abbreviated to moment). It represents the capability of a force to produce change in the rotational motion of the body. The concept originated with the studies by Archimedes of the usage of levers, which is reflected in his famous quote: "''Give me a lever and a place to stand and I will move the Earth''". Just as a linear force is a push or a pull, a torque can be thought of as a twist to an object around a specific axis. Torque is defined as the product of the magnitude of the perpendicular component of the force and the distance of the line of action of a force from the point around which it is being determined. The law of conservation of energy can also be used to understand torque. The symbol for torque is typically \boldsymbol\tau, the lowercase Greek letter ''tau''. When being referred to as moment of force, it is commonly denoted by . I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shale
Shale is a fine-grained, clastic sedimentary rock formed from mud that is a mix of flakes of clay minerals (hydrous aluminium phyllosilicates, e.g. kaolin, Al2 Si2 O5( OH)4) and tiny fragments (silt-sized particles) of other minerals, especially quartz and calcite.Blatt, Harvey and Robert J. Tracy (1996) ''Petrology: Igneous, Sedimentary and Metamorphic'', 2nd ed., Freeman, pp. 281–292 Shale is characterized by its tendency to split into thin layers ( laminae) less than one centimeter in thickness. This property is called '' fissility''. Shale is the most common sedimentary rock. The term ''shale'' is sometimes applied more broadly, as essentially a synonym for mudrock, rather than in the more narrow sense of clay-rich fissile mudrock. Texture Shale typically exhibits varying degrees of fissility. Because of the parallel orientation of clay mineral flakes in shale, it breaks into thin layers, often splintery and usually parallel to the otherwise indistinguishable bedding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Chloride
Calcium chloride is an inorganic compound, a salt with the chemical formula . It is a white crystalline solid at room temperature, and it is highly soluble in water. It can be created by neutralising hydrochloric acid with calcium hydroxide. Calcium chloride is commonly encountered as a hydrated solid with generic formula , where ''n'' = 0, 1, 2, 4, and 6. These compounds are mainly used for de-icing and dust control. Because the anhydrous salt is hydroscopic and deliquescent, it is used as a desiccant.Robert Kemp, Suzanne E. Keegan "Calcium Chloride" in Ullmann's Encyclopedia of Industrial Chemistry 2000, Wiley-VCH, Weinheim. Uses De-icing and freezing-point depression By depressing the freezing point of water, calcium chloride is used to prevent ice formation and is used to de-ice. This application consumes the greatest amount of calcium chloride. Calcium chloride is relatively harmless to plants and soil. As a deicing agent, it is much more effective at lower temperat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in soap manufacture, and sodium chloride (edible salt) is a de-icing agent and a nutrient for animals inc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Freshwater
Fresh water or freshwater is any naturally occurring liquid or frozen water containing low concentrations of dissolved salts and other total dissolved solids. Although the term specifically excludes seawater and brackish water, it does include non- salty mineral-rich waters such as chalybeate springs. Fresh water may encompass frozen and meltwater in ice sheets, ice caps, glaciers, snowfields and icebergs, natural precipitations such as rainfall, snowfall, hail/ sleet and graupel, and surface runoffs that form inland bodies of water such as wetlands, ponds, lakes, rivers, streams, as well as groundwater contained in aquifers, subterranean rivers and lakes. Fresh water is the water resource that is of the most and immediate use to humans. Water is critical to the survival of all living organisms. Many organisms can thrive on salt water, but the great majority of higher plants and most insects, amphibians, reptiles, mammals and birds need fresh water to survive. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aniline Point
The aniline point of an oil is defined as the minimum temperature at which equal volumes of aniline () and lubricant oil are miscible, i.e. form a single phase upon mixing. The value gives an approximation for the content of aromatic compounds in the oil, since the miscibility of aniline, which is also an aromatic compound suggests the presence of similar (i.e. aromatic) compounds in the oil. The lower the aniline point, the greater is the content of aromatic compounds in the oil. The aniline point serves as a reasonable proxy for aromaticity of oils consisting mostly of saturated hydrocarbons (i.e. alkanes, paraffins) or unsaturated compounds (mostly aromatics). Significant chemical functionalization of the oil (chlorination, sulfonation, etc.) can interfere with the measurement, due to changes to the solvency of the functionalized oil. Aniline point indicates if an oil is likely to damage elastomers (rubber compounds) that come in contact with the oil. Determination of aniline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flash Point
The flash point of a material is the "lowest liquid temperature at which, under certain standardized conditions, a liquid gives off vapours in a quantity such as to be capable of forming an ignitable vapour/air mixture". (EN 60079-10-1) The flash point is sometimes confused with the autoignition temperature, the temperature that causes spontaneous ignition. The fire point is the lowest temperature at which the vapors keep burning after the ignition source is removed. It is higher than the flash point, because at the flash point vapor may not be produced fast enough to sustain combustion. Neither flash point nor fire point depends directly on the ignition source temperature, but ignition source temperature is far higher than either the flash or fire point. Fuels The flash point is a descriptive characteristic that is used to distinguish between flammable fuels, such as petrol (also known as gasoline), and combustible fuels, such as diesel. It is also used to characterize th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |