|
O-linked Glycosylation
''O''-linked glycosylation is the attachment of a sugar molecule to the oxygen atom of serine (Ser) or threonine (Thr) residues in a protein. ''O''-glycosylation is a post-translational modification that occurs after the protein has been synthesised. In eukaryotes, it occurs in the endoplasmic reticulum, Golgi apparatus and occasionally in the cytoplasm; in prokaryotes, it occurs in the cytoplasm. Several different sugars can be added to the serine or threonine, and they affect the protein in different ways by changing protein stability and regulating protein activity. O-glycans, which are the sugars added to the serine or threonine, have numerous functions throughout the body, including trafficking of cells in the immune system, allowing recognition of foreign material, controlling cell metabolism and providing cartilage and tendon flexibility. Because of the many functions they have, changes in O-glycosylation are important in many diseases including cancer, diabetes and Alzheime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double sugars, are molecules made of two bonded monosaccharides; common examples are sucrose (glucose + fructose), lactose (glucose + galactose), and maltose (two molecules of glucose). White sugar is a refined form of sucrose. In the body, compound sugars are hydrolysed into simple sugars. Longer chains of monosaccharides (>2) are not regarded as sugars, and are called oligosaccharides or polysaccharides. Starch is a glucose polymer found in plants, the most abundant source of energy in human food. Some other chemical substances, such as glycerol and sugar alcohols, may have a sweet taste, but are not classified as sugar. Sugars are found in the tissues of most plants. Honey and fruits are abundant natural sources of simple su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leukocyte
White blood cells, also called leukocytes or leucocytes, are the cells of the immune system that are involved in protecting the body against both infectious disease and foreign invaders. All white blood cells are produced and derived from multipotent cells in the bone marrow known as hematopoietic stem cells. Leukocytes are found throughout the body, including the blood and lymphatic system. All white blood cells have nuclei, which distinguishes them from the other blood cells, the anucleated red blood cells (RBCs) and platelets. The different white blood cells are usually classified by cell lineage (myeloid cells or lymphoid cells). White blood cells are part of the body's immune system. They help the body fight infection and other diseases. Types of white blood cells are granulocytes (neutrophils, eosinophils, and basophils), and agranulocytes (monocytes, and lymphocytes (T cells and B cells)). Myeloid cells ( myelocytes) include neutrophils, eosinophils, mast cells, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PSGL-1 Structure Showing O-glycans Present
Selectin P ligand, also known as SELPLG or CD162 (cluster of differentiation 162), is a human gene. SELPLG codes for PSGL-1, the high affinity counter-receptor for P-selectin on myeloid cells and stimulated T lymphocytes. As such, it plays a critical role in the tethering of these cells to activated platelets or endothelia expressing P-selectin. The organization of the SELPLG gene closely resembles that of CD43 and the human platelet glycoprotein GpIb-alpha both of which have an intron in the 5-prime-noncoding region, a long second exon containing the complete coding region, and TATA-less promoters. P-selectin glycoprotein ligand-1 (PSGL-1) is a glycoprotein found on white blood cells and endothelial cells that binds to P-selectin (P stands for platelet), which is one of a family of selectins that includes E-selectin (endothelial) and L-selectin (leukocyte). Selectins are part of the broader family of cell adhesion molecules. PSGL-1 can bind to all three members of the family bu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lectins
Lectins are carbohydrate-binding proteins that are highly specific for sugar groups that are part of other molecules, so cause agglutination of particular cells or precipitation of glycoconjugates and polysaccharides. Lectins have a role in recognition at the cellular and molecular level and play numerous roles in biological recognition phenomena involving cells, carbohydrates, and proteins. Lectins also mediate attachment and binding of bacteria, viruses, and fungi to their intended targets. Lectins are ubiquitous in nature and are found in many foods. Some foods, such as beans and grains, need to be cooked, fermented or sprouted to reduce lectin content. Some lectins are beneficial, such as CLEC11A, which promotes bone growth, while others may be powerful toxins such as ricin. Lectins may be disabled by specific mono- and oligosaccharides, which bind to ingested lectins from grains, legumes, nightshade plants, and dairy; binding can prevent their attachment to the carbohyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Precursor (chemistry)
In chemistry, a precursor is a compound that participates in a chemical reaction that produces another compound. In biochemistry, the term "precursor" often refers more specifically to a chemical compound preceding another in a metabolic pathway, such as a protein precursor. Illicit drug precursors In 1988, the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances introduced detailed provisions and requirements relating the control of precursors used to produce drugs of abuse. In Europe the Regulation (EC) No. 273/2004 of the European Parliament and of the Council on drug precursors was adopted on 11 February 2004. (European law on drug precursors) Illicit explosives precursors On January 15, 2013, the Regulation (EU) No. 98/2013 of the European Parliament and of the Council on the marketing and use of explosives precursors was adopted. The Regulation harmonises rules across Europe on the making available, introduction, possession and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sugars That Form The H, A And B Antigens
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double sugars, are molecules made of two bonded monosaccharides; common examples are sucrose (glucose + fructose), lactose (glucose + galactose), and maltose (two molecules of glucose). White sugar is a refined form of sucrose. In the body, compound sugars are hydrolysed into simple sugars. Longer chains of monosaccharides (>2) are not regarded as sugars, and are called oligosaccharides or polysaccharides. Starch is a glucose polymer found in plants, the most abundant source of energy in human food. Some other chemical substances, such as glycerol and sugar alcohols, may have a sweet taste, but are not classified as sugar. Sugars are found in the tissues of most plants. Honey and fruits are abundant natural sources of simple sugars. Sucrose ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sialic Acid
Sialic acids are a class of alpha-keto acid sugars with a nine-carbon backbone. The term "sialic acid" (from the Greek for saliva, - ''síalon'') was first introduced by Swedish biochemist Gunnar Blix in 1952. The most common member of this group is ''N''-acetylneuraminic acid (Neu5Ac or NANA) found in animals and some prokaryotes. Sialic acids are found widely distributed in animal tissues and related forms are found to a lesser extent in other organisms like in some micro-algae, bacteria and archaea. Sialic acids are commonly part of glycoproteins, glycolipids or gangliosides, where they decorate the end of sugar chains at the surface of cells or soluble proteins. However, sialic acids have been also observed in ''Drosophila'' embryos and other insects. Generally, plants seem not to contain or display sialic acids. In humans the brain has the highest sialic acid content, where these acids play an important role in neural transmission and ganglioside structure in synaptog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fucose
Fucose is a hexose deoxy sugar with the chemical formula C6H12O5. It is found on ''N''-linked glycans on the mammalian, insect and plant cell surface. Fucose is the fundamental sub-unit of the seaweed polysaccharide fucoidan. The α(1→3) linked core of fucose is a suspected carbohydrate antigen for IgE-mediated allergy. Two structural features distinguish fucose from other six-carbon sugars present in mammals: the lack of a hydroxyl group on the carbon at the 6-position (C-6) (thereby making it a deoxy sugar) and the L -configuration. It is equivalent to 6-deoxy--galactose. In the fucose-containing glycan structures, fucosylated glycans, fucose can exist as a terminal modification or serve as an attachment point for adding other sugars. In human ''N''-linked glycans, fucose is most commonly linked α-1,6 to the reducing terminal β-''N''-acetylglucosamine. However, fucose at the non-reducing termini linked α-1,2 to galactose forms the H antigen, the substructure of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-acetylglucosamine
''N''-Acetylglucosamine (GlcNAc) is an amide derivative of the monosaccharide glucose. It is a secondary amide between glucosamine and acetic acid. It is significant in several biological systems. It is part of a biopolymer in the bacterial cell wall, which is built from alternating units of GlcNAc and ''N''-acetylmuramic acid (MurNAc), cross-linked with oligopeptides at the lactic acid residue of MurNAc. This layered structure is called peptidoglycan (formerly called murein). GlcNAc is the monomeric unit of the polymer chitin, which forms the exoskeletons of arthropods like insects and crustaceans. It is the main component of the radulas of mollusks, the beaks of cephalopods, and a major component of the cell walls of most fungi. Polymerized with glucuronic acid, it forms hyaluronan. GlcNAc has been reported to be an inhibitor of elastase release from human polymorphonuclear leukocytes (range 8–17% inhibition), however this is much weaker than the inhibition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galactose
Galactose (, '' galacto-'' + ''-ose'', "milk sugar"), sometimes abbreviated Gal, is a monosaccharide sugar that is about as sweet as glucose, and about 65% as sweet as sucrose. It is an aldohexose and a C-4 epimer of glucose. A galactose molecule linked with a glucose molecule forms a lactose molecule. Galactan is a polymeric form of galactose found in hemicellulose, and forming the core of the galactans, a class of natural polymeric carbohydrates. D-Galactose is also known as brain sugar since it is a component of glycoproteins (oligosaccharide-protein compounds) found in nerve tissue. Etymology The word ''galactose'' was coined by Charles Weissman in the mid-19th century and is derived from Greek ''galaktos'' (of milk) and the generic chemical suffix for sugars ''-ose''. The etymology is comparable to that of the word ''lactose'' in that both contain roots meaning "milk sugar". Lactose is a disaccharide of galactose plus glucose. Structure and isomerism Galactose exists in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
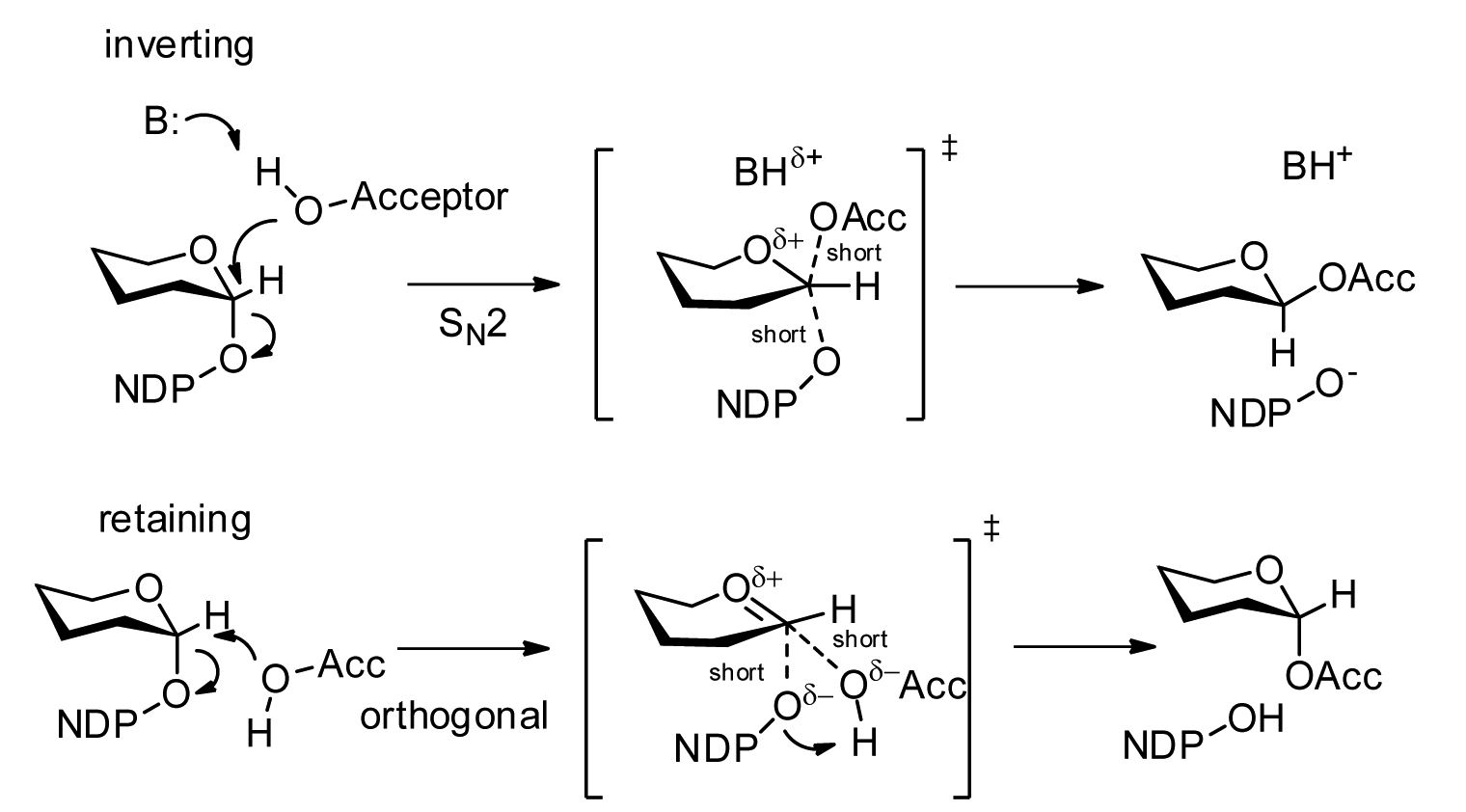
Glycosyltransferases
Glycosyltransferases (GTFs, Gtfs) are enzymes ( EC 2.4) that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar (also known as the " glycosyl donor") to a nucleophilic glycosyl acceptor molecule, the nucleophile of which can be oxygen- carbon-, nitrogen-, or sulfur-based. The result of glycosyl transfer can be a carbohydrate, glycoside, oligosaccharide, or a polysaccharide. Some glycosyltransferases catalyse transfer to inorganic phosphate or water. Glycosyl transfer can also occur to protein residues, usually to tyrosine, serine, or threonine to give O-linked glycoproteins, or to asparagine to give N-linked glycoproteins. Mannosyl groups may be transferred to tryptophan to generate C-mannosyl tryptophan, which is relatively abundant in eukaryotes. Transferases may also use lipids as an acceptor, forming glycolipids, and even use lipid-linked sugar phosphate donors, such as dolichol phosphates in euk ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |