|
Nicholas Reaction
The Nicholas reaction is an organic reaction where a dicobalt octacarbonyl-stabilized propargylic cation is reacted with a nucleophile. Oxidative demetallation gives the desired alkylated alkyne. It is named after Kenneth M. Nicholas. Several reviews have been published. Reaction mechanism The addition of dicobalt octacarbonyl to a propargylic ether (1) gives the dicobalt intermediate 2. Reaction with HBF4 or Lewis acid gives the key dicobalt octacarbonyl-stabilized propargylic cation (3a and 3b). Addition of a nucleophile followed by a mild oxidation gives the desired substituted alkyne (5). The likely intermediates in the reactions, propargylium)Co2(CO)6sup>+ cation 3, possessed considerable stability. It was, in fact, possible to observe these cations by 1H NMR at 10 °C when generated using ''d''-trifluoroacetic acid. Later, Richard E. Connor and Nicholas were able to isolate salts of such cations 3 as stable, dark red solids by treatment of the Co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels-Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic chemistry has a strong tradition of naming a spe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin. Structure and bonding Ethers feature bent C–O–C linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of oxygen in ethers, alcohols, and water ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallic Chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term " metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pauson–Khand Reaction
The Pauson–Khand reaction (or PKR or PK-type reaction) is a chemical reaction described as a 2+2+1.html" ;"title="/nowiki>2+2+1">/nowiki>2+2+1/nowiki> cycloaddition between an alkyne, an alkene and carbon monoxide to form a α,β- cyclopentenone. Ihsan Ullah Khand (1935-1980) discovered the reaction around 1970, while working as a postdoctoral associate with Peter Ludwig Pauson (1925–2013) at the University of Strathclyde in Glasgow. Pauson and Khand's initial findings were intermolecular in nature, but starting a decade after the reaction's discovery, many intramolecular examples have been highlighted in both synthesis and methodology reports. This reaction was originally mediated by stoichiometric amounts of dicobalt octacarbonyl, but newer versions are both more efficient, enhancing reactivity and yield via utilizing different chiral auxiliaries for stereo induction, main group transition-metals (Ti, Mo, W, Fe, Co, Ni, Ru, Rh, Ir and Pd), and additives. Mechanism W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orbital Hybridisation
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed out that a carbon atom forms fou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
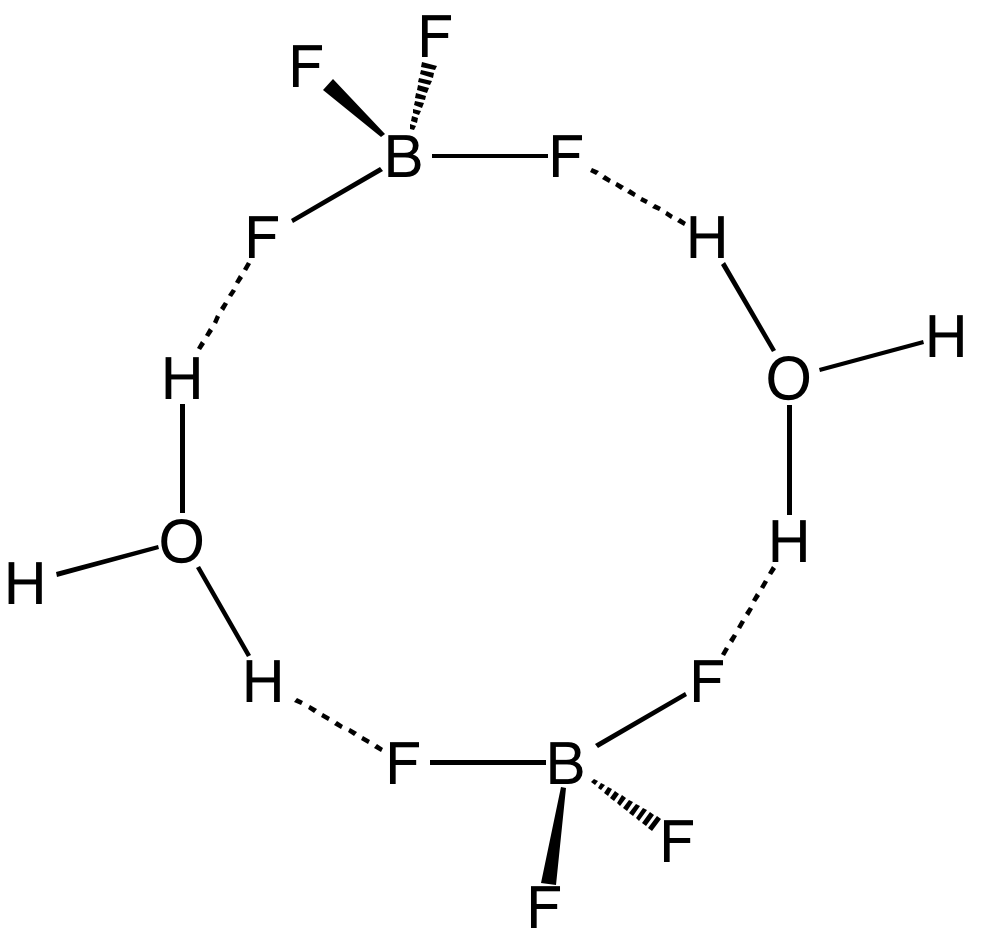
Tetrafluoroboric Acid
Fluoroboric acid or tetrafluoroboric acid (archaically, fluoboric acid) is an inorganic compound with the chemical formula +BF4−], where H+ represents the solvated proton. The solvent can be any suitably Lewis-basic entity. For instance, in water, it can be represented by (oxonium tetrafluoroborate), although more realistically, several water molecules solvate the proton: (H2O)''n''+BF4−]. The ethyl ether solvate is also commercially available: (Et2O)''n''+BF4−], where ''n'' is most likely 2. Unlike other strong acids like H2SO4 or HClO4, the pure unsolvated substance does not exist (see below). It is mainly produced as a precursor to other fluoroborate salts.Gregory K. Friestad, Bruce P. Branchaud "Tetrafluoroboric Acid" E-Eros Encyclopedia of Reagents for Organic Synthesis. It is a strong acid. Fluoroboric acid is corrosive and attacks the skin. It is available commercially as a solution in water and other solvents such as diethyl ether. It is a strong acid with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoroantimonic Acid
Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions (the simplest being and ). This substance is a superacid that can be over a billion times stronger than 100% pure sulfuric acid in terms of its protonating ability measured by Hammett function. It even protonates some hydrocarbons to afford pentacoordinate carbocations ( carbonium ions). Fluoroantimonic acid is corrosive. For example, it cannot be contained directly in glass carboys, as it attacks glass, but can be stored in containers lined with PTFE (Teflon). Chemical composition Fluoroantimonic acid is formed by combining hydrogen fluoride and antimony pentafluoride: :SbF5 + 2 HF + H2F+ The speciation (i.e., the inventory of components) of "fluoroantimonic acid" is complex. Spectroscopic measurements show that fluoroantimonic acid consists of a mixture of HF-solvated protons, –_(such_as_)._Thus,_the_formula_""_is_a_convenient_but_over ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoroacetic Acid
Trifluoroacetic acid (TFA) is an organofluorine compound with the chemical formula CF3CO2H. It is a structural analogue of acetic acid with all three of the acetyl group's hydrogen atoms replaced by fluorine atoms and is a colorless liquid with a vinegar-like odor. TFA is a stronger acid than acetic acid, having an acid ionisation constant, ''K''a, that is approximately 34,000 times higher, as the highly electronegative fluorine atoms and consequent electron-withdrawing nature of the trifluoromethyl group weakens the oxygen-hydrogen bond (allowing for greater acidity) and stabilises the anionic conjugate base. TFA is widely used in organic chemistry for various purposes. Synthesis TFA is prepared industrially by the electrofluorination of acetyl chloride or acetic anhydride, followed by hydrolysis of the resulting trifluoroacetyl fluoride: : + 4 → + 3 + : + → + Where desired, this compound may be dried by addition of trifluoroacetic anhydride. An older ro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton Nuclear Magnetic Resonance
Proton nuclear magnetic resonance (proton NMR, hydrogen-1 NMR, or 1H NMR) is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen (H) is used, practically all the hydrogen consists of the isotope 1H (hydrogen-1; i.e. having a proton for a nucleus). Simple NMR spectra are recorded in solution, and solvent protons must not be allowed to interfere. Deuterated (deuterium = 2H, often symbolized as D) solvents especially for use in NMR are preferred, e.g. deuterated water, D2O, deuterated acetone, (CD3)2CO, deuterated methanol, CD3OD, deuterated dimethyl sulfoxide, (CD3)2SO, and deuterated chloroform, CDCl3. However, a solvent without hydrogen, such as carbon tetrachloride, CCl4 or carbon disulfide, CS2, may also be used. Historically, deuterated solvents were supplied with a small amount (typically 0.1%) of tet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Intermediate
In chemistry, a reaction intermediate or an intermediate is a molecular entity that is formed from the reactants (or preceding intermediates) but is consumed in further reactions in stepwise chemical reactions that contain multiple elementary steps. Intermediates are the reaction product of one elementary step, but do not appear in the chemical equation for an overall chemical equation. For example, consider this hypothetical stepwise reaction: :A + B -> C + D The reaction includes two elementary steps: :A + B -> X :X -> C + D In this example, X is a reaction intermediate. IUPAC definition The IUPAC Gold Book defines an ''intermediate'' as a compound that has a lifetime greater than a molecular vibration that is formed (directly or indirectly) from the reactants and reacts further to give (either directly or indirectly) the products of a chemical reaction. The lifetime condition distinguishes true, chemically distinct intermediates from vibrational states or such transition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane (Me3B) is a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis. From p. 142: "We are inclined to think of substances as poss ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicholas Reaction Mechanism
Nicholas is a male given name and a surname. The Eastern Orthodox Church, the Roman Catholic Church, and the Anglican Churches celebrate Saint Nicholas every year on December 6, which is the name day for "Nicholas". In Greece, the name and its derivatives are especially popular in maritime regions, as St. Nicholas is considered the protector saint of seafarers. Origins The name is derived from the Greek name Νικόλαος (''Nikolaos''), understood to mean 'victory of the people', being a compound of νίκη ''nikē'' 'victory' and λαός ''laos'' 'people'.. An ancient paretymology of the latter is that originates from λᾶς ''las'' ( contracted form of λᾶας ''laas'') meaning 'stone' or 'rock', as in Greek mythology, Deucalion and Pyrrha recreated the people after they had vanished in a catastrophic deluge, by throwing stones behind their shoulders while they kept marching on. The name became popular through Saint Nicholas, Bishop of Myra in Lycia, the inspir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |