|
Muse Cell
A Muse cell (Multi-lineage differentiating stress enduring cell) is an endogenous non-cancerous pluripotent stem cell. They reside in the connective tissue of nearly every organ including the umbilical cord, bone marrow and peripheral blood. They are collectable from commercially obtainable mesenchymal cells such as human fibroblasts, bone marrow-mesenchymal stem cells and adipose-derived stem cells. Muse cells are able to generate cells representative of all three germ layers from a single cell both spontaneously and under cytokine induction. Expression of pluripotency genes and triploblastic differentiation are self-renewable over generations. Muse cells do not undergo teratoma formation when transplanted into a host environment in vivo. This can be explained in part by their intrinsically low telomerase activity, eradicating the risk of tumorigenesis through unbridled cell proliferation. They were discovered in 2010 by Mari Dezawa and her research group. Clinical trials for acute ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
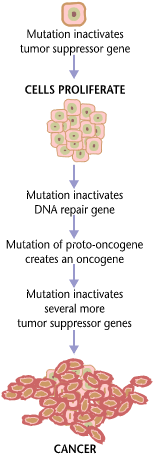
Carcinogenesis
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abnormal cell division. Cell division is a physiological process that occurs in almost all tissues and under a variety of circumstances. Normally, the balance between proliferation and programmed cell death, in the form of apoptosis, is maintained to ensure the integrity of tissues and organs. According to the prevailing accepted theory of carcinogenesis, the somatic mutation theory, mutations in DNA and epimutations that lead to cancer disrupt these orderly processes by interfering with the programming regulating the processes, upsetting the normal balance between proliferation and cell death. This results in uncontrolled cell division and the evolution of those cells by natural selection in the body. Only certain mutations lead to canc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosinase
Tyrosinase is an oxidase that is the rate-limiting enzyme for controlling the production of melanin. The enzyme is mainly involved in two distinct reactions of melanin synthesis otherwise known as the Raper Mason pathway. Firstly, the hydroxylation of a monophenol and secondly, the conversion of an o-diphenol to the corresponding o-quinone. o-Quinone undergoes several reactions to eventually form melanin. Tyrosinase is a copper-containing enzyme present in plant and animal tissues that catalyzes the production of melanin and other pigments from tyrosine by oxidation. It is found inside melanosomes which are synthesized in the skin melanocytes. In humans, the tyrosinase enzyme is encoded by the ''TYR'' gene. Catalyzed reaction Tyrosinase carries out the oxidation of phenols such as tyrosine and dopamine using dioxygen (O2). In the presence of catechol, benzoquinone is formed (see reaction below). Hydrogens removed from catechol combine with oxygen to form water. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melanocyte
Melanocytes are melanin-producing neural crest-derived cells located in the bottom layer (the stratum basale) of the skin's epidermis, the middle layer of the eye (the uvea), the inner ear, vaginal epithelium, meninges, bones, and heart. Melanin is a dark pigment primarily responsible for skin color. Once synthesized, melanin is contained in special organelles called melanosomes which can be transported to nearby keratinocytes to induce pigmentation. Thus darker skin tones have more melanosomes present than lighter skin tones. Functionally, melanin serves as protection against UV radiation. Melanocytes also have a role in the immune system. Function Through a process called melanogenesis, melanocytes produce melanin, which is a pigment found in the skin, eyes, hair, nasal cavity, and inner ear. This melanogenesis leads to a long-lasting pigmentation, which is in contrast to the pigmentation that originates from oxidation of already-existing melanin. There a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MAP-2
Microtubule-associated protein 2 is a protein in humans that is encoded by the ''MAP2'' gene. Function This gene encodes a protein that belongs to the microtubule-associated protein family. The proteins of this family were originally isolated since they copurify with tubulin in polymerization experiments: tubulin in cell extracts can be made to polymerize to produce microtubules (MT) under the influence of heat and the addition of GTP, and the MT can then be collected by centrifugation. When this is done a series of microtubule associated proteins are collected along with the MT and can be detected by SDS-PAGE and other methods. Brain extracts are rich in several of these proteins, MAP2 being one of these. The single MAP2 gene produces four major transcripts producing four proteins, MAP2A, MAP2B, MAP2C and MAP2D. MAP2A and MAP2B are very high molecular weight proteins, with apparent molecular weight on SDS-PAGE about 250kDa, while MAP2C and MAP2D are much lower molecular weight ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurofilament
Neurofilaments (NF) are classed as type IV intermediate filaments found in the cytoplasm of neurons. They are protein polymers measuring 10 nm in diameter and many micrometers in length. Together with microtubules (~25 nm) and microfilaments (7 nm), they form the neuronal cytoskeleton. They are believed to function primarily to provide structural support for axons and to regulate axon diameter, which influences nerve conduction velocity. The proteins that form neurofilaments are members of the intermediate filament protein family, which is divided into six types based on their gene organization and protein structure. Types I and II are the keratins which are expressed in epithelia. Type III contains the proteins vimentin, desmin, peripherin and glial fibrillary acidic protein (GFAP). Type IV consists of the neurofilament proteins L, M, H and internexin. Type V consists of the Lamin, nuclear lamins, and type VI consists of the protein Nestin (protein), nesti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Musashi (protein)
is a Japanese name, which may refer to: People *, Japanese master swordsman, painter, and author of ''The Book of Five Rings'' *, Japanese science fiction writer *, Japanese former professional footballer *, Japanese retired kickboxer *, Wushu martial artist and actor *, Japanese footballer Places * Musashi Province, an old province of Japan * Musashi Imperial Graveyard * Musashi, Ōita, Japan * Musashi University * Musashi-Kosugi Station Science * Musashi-1, RNA-binding protein Musashi homolog 1 * Musashi-2, RNA-binding protein Musashi homolog 2 Ships * List of ships named ''Musashi'' Entertainment * ''Musashi'' (novel), a 1935 novel by Eiji Yoshikawa * Musashi's, a Japanese feline musical group * ''Brave Fencer Musashi'', a 1998 PlayStation video game * '' Musashi: Samurai Legend'', a 2005 PlayStation 2 video game Characters * Joe Musashi, the protagonist of the ''Shinobi'' video games * Musashi, the protagonist of the video games ''Brave Fencer Musashi' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NeuroD
NeuroD, also called Beta2, is a basic helix-loop-helix transcription factor expressed in certain parts of brain, beta pancreatic cells and enteroendocrine cells. It is involved in the differentiation of nervous system and development of pancreas. It heterodimerizes with the products of E2A gene and controls the transcription of a variety of genes by identifying and binding E boxes in their promoter region. In rodents NeuroD is involved in the development of the retina The retina (from la, rete "net") is the innermost, light-sensitive layer of tissue of the eye of most vertebrates and some molluscs. The optics of the eye create a focused two-dimensional image of the visual world on the retina, which the .... In mammals there are two types of this factor: * NeuroD1 * NeuroD2 * NeuroD4 * NeuroD6 References Transcription factors {{biochemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nestin (protein)
Nestin is a protein that in humans is encoded by the NES gene. Nestin (acronym for neuroepithelial stem cell protein) is a type VI intermediate filament (IF) protein. These intermediate filament proteins are expressed mostly in nerve cells where they are implicated in the radial growth of the axon. Seven genes encode for the heavy (NF-H), medium (NF-M) and light neurofilament (NF-L) proteins, nestin and α-internexin in nerve cells, synemin α and desmuslin/synemin β (two alternative transcripts of the DMN gene) in muscle cells, and syncoilin (also in muscle cells). Members of this group mostly preferentially coassemble as heteropolymers in tissues. Steinert et al. has shown that nestin forms homodimers and homotetramers but does not form IF by itself in vitro. In mixtures, nestin preferentially co-assembles with purified vimentin or the type IV IF protein internexin to form heterodimer coiled-coil molecules. Gene Structurally, nestin has the shortest head domain (N-terminus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endothelial Progenitor Cell
Endothelial progenitor cell (or EPC) is a term that has been applied to multiple different cell types that play roles in the regeneration of the endothelial lining of blood vessels. Outgrowth endothelial cells are an EPC subtype committed to endothelial cell formation. Despite the history and controversy, the EPC in all its forms remains a promising target of regenerative medicine research. History and controversy Developmentally, the endothelium arises in close contact with the hematopoietic system. This, and the existence of hemogenic endothelium, led to a belief and search for adult hemangioblast- or angioblast-like cells; cells which could give rise to functional vasculature in adults. The existence of endothelial progenitor cells has been posited since the mid-twentieth century, however their existence was not confirmed until the 1990s when Asahara et al. published the discovery of the first putative EPC. Recently, controversy has developed over the definition of true endoth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Von Willebrand Factor
Von Willebrand factor (VWF) () is a blood glycoprotein involved in hemostasis, specifically, platelet adhesion. It is deficient and/or defective in von Willebrand disease and is involved in many other diseases, including thrombotic thrombocytopenic purpura, Heyde's syndrome, and possibly hemolytic–uremic syndrome. Increased plasma levels in many cardiovascular, neoplastic, metabolic (e.g. diabetes), and connective tissue diseases are presumed to arise from adverse changes to the endothelium, and may predict an increased risk of thrombosis. Biochemistry Synthesis VWF is a large multimeric glycoprotein present in blood plasma and produced constitutively as ultra-large VWF in endothelium (in the Weibel–Palade bodies), megakaryocytes (α-granules of platelets), and subendothelial connective tissue. Structure The basic VWF monomer is a 2050-amino acid protein. Every monomer contains a number of specific domains with a specific function; elements of note are: * the D'/D3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CD31
Platelet endothelial cell adhesion molecule (PECAM-1) also known as cluster of differentiation 31 (CD31) is a protein that in humans is encoded by the ''PECAM1'' gene found on chromosome17q23.3. PECAM-1 plays a key role in removing aged neutrophils from the body. Structure PECAM-1 is a highly glycosylated protein with a mass of approximately 130 kDa. The structure of this protein was determined by molecular cloning in 1990, when it was found out that PECAM-1 has N-terminal domain with 574 amino acids, transmembrane domain with 19 amino acids and C-terminal cytoplasmic domain with 118 amino acids. The N-terminal domain consists of six extracellular Ig-like domains. Interactions PECAM-1 is a cell-cell adhesion protein which interacts with other PECAM-1 molecules through homophilic interactions or with non-PECAM-1 molecules through heterophilic interactions''.'' Homophilic interactions between PECAM-1 molecules are mediated by antiparallel interactions between extracellular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |