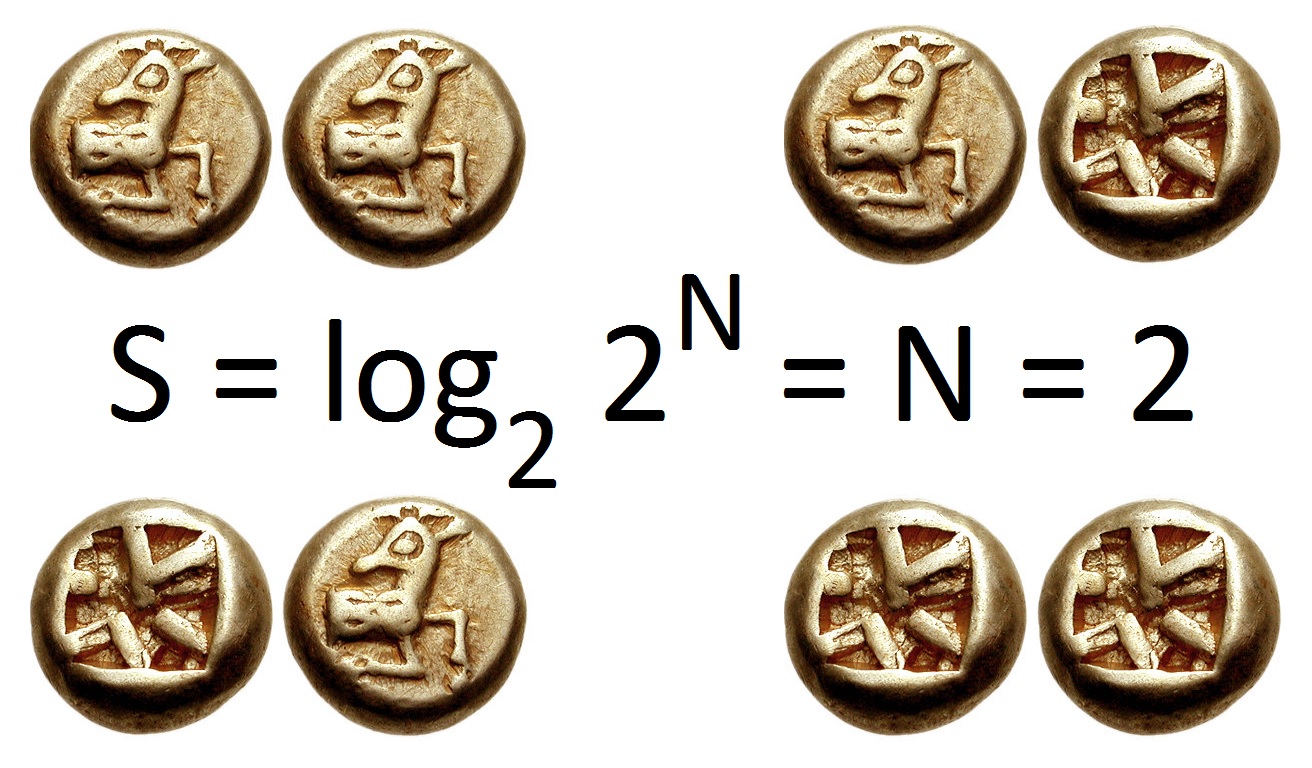|
Maximum Entropy Thermodynamics
In physics, maximum entropy thermodynamics (colloquially, ''MaxEnt'' thermodynamics) views equilibrium thermodynamics and statistical mechanics as inference processes. More specifically, MaxEnt applies inference techniques rooted in Shannon information theory, Bayesian probability, and the principle of maximum entropy. These techniques are relevant to any situation requiring prediction from incomplete or insufficient data (e.g., image reconstruction, signal processing, spectral analysis, and inverse problems). MaxEnt thermodynamics began with two papers by Edwin T. Jaynes published in the 1957 ''Physical Review''. Maximum Shannon entropy Central to the MaxEnt thesis is the principle of maximum entropy. It demands as given some partly specified model and some specified data related to the model. It selects a preferred probability distribution to represent the model. The given data state "testable information" about the probability distribution, for example particular expectat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physics
Physics is the natural science that studies matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which relates to the order of nature, or, in other words, to the regular succession of events." Physics is one of the most fundamental scientific disciplines, with its main goal being to understand how the universe behaves. "Physics is one of the most fundamental of the sciences. Scientists of all disciplines use the ideas of physics, including chemists who study the structure of molecules, paleontologists who try to reconstruct how dinosaurs walked, and climatologists who study how human activities affect the atmosphere and oceans. Physics is also the foundation of all engineering and technology. No engineer could design a flat-screen TV, an interplanetary spacecraft, or even a better mousetrap without first understanding the basic laws of physic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Statistical Ensemble
In physics, specifically statistical mechanics, an ensemble (also statistical ensemble) is an idealization consisting of a large number of virtual copies (sometimes infinitely many) of a system, considered all at once, each of which represents a possible state that the real system might be in. In other words, a statistical ensemble is a set of systems of particles used in statistical mechanics to describe a single system. The concept of an ensemble was introduced by J. Willard Gibbs in 1902. A thermodynamic ensemble is a specific variety of statistical ensemble that, among other properties, is in statistical equilibrium (defined below), and is used to derive the properties of thermodynamic systems from the laws of classical or quantum mechanics. Physical considerations The ensemble formalises the notion that an experimenter repeating an experiment again and again under the same macroscopic conditions, but unable to control the microscopic details, may expect to observe a ran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Differential Entropy
Differential entropy (also referred to as continuous entropy) is a concept in information theory that began as an attempt by Claude Shannon to extend the idea of (Shannon) entropy, a measure of average surprisal of a random variable, to continuous probability distributions. Unfortunately, Shannon did not derive this formula, and rather just assumed it was the correct continuous analogue of discrete entropy, but it is not. The actual continuous version of discrete entropy is the limiting density of discrete points (LDDP). Differential entropy (described here) is commonly encountered in the literature, but it is a limiting case of the LDDP, and one that loses its fundamental association with discrete entropy. In terms of measure theory, the differential entropy of a probability measure is the negative relative entropy from that measure to the Lebesgue measure, where the latter is treated as if it were a probability measure, despite being unnormalized. Definition Let X be a rando ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluctuation Theorem
The fluctuation theorem (FT), which originated from statistical mechanics, deals with the relative probability that the entropy of a system which is currently away from thermodynamic equilibrium (i.e., maximum entropy) will increase or decrease over a given amount of time. While the second law of thermodynamics predicts that the entropy of an isolated system should tend to increase until it reaches equilibrium, it became apparent after the discovery of statistical mechanics that the second law is only a statistical one, suggesting that there should always be some nonzero probability that the entropy of an isolated system might spontaneously ''decrease''; the fluctuation theorem precisely quantifies this probability. Statement Roughly, the fluctuation theorem relates to the probability distribution of the time-averaged irreversible entropy production, denoted \overline_t. The theorem states that, in systems away from equilibrium over a finite time ''t'', the ratio between the probab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Onsager Reciprocal Relations
In thermodynamics, the Onsager reciprocal relations express the equality of certain ratios between flows and forces in thermodynamic systems out of equilibrium, but where a notion of local equilibrium exists. "Reciprocal relations" occur between different pairs of forces and flows in a variety of physical systems. For example, consider fluid systems described in terms of temperature, matter density, and pressure. In this class of systems, it is known that temperature differences lead to heat flows from the warmer to the colder parts of the system; similarly, pressure differences will lead to matter flow from high-pressure to low-pressure regions. What is remarkable is the observation that, when both pressure and temperature vary, temperature differences at constant pressure can cause matter flow (as in convection) and pressure differences at constant temperature can cause heat flow. Perhaps surprisingly, the heat flow per unit of pressure difference and the density (matter) flow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-equilibrium Thermodynamics
Non-equilibrium thermodynamics is a branch of thermodynamics that deals with physical systems that are not in thermodynamic equilibrium but can be described in terms of macroscopic quantities (non-equilibrium state variables) that represent an extrapolation of the variables used to specify the system in thermodynamic equilibrium. Non-equilibrium thermodynamics is concerned with transport processes and with the rates of chemical reactions. Almost all systems found in nature are not in thermodynamic equilibrium, for they are changing or can be triggered to change over time, and are continuously and discontinuously subject to flux of matter and energy to and from other systems and to chemical reactions. Some systems and processes are, however, in a useful sense, near enough to thermodynamic equilibrium to allow description with useful accuracy by currently known non-equilibrium thermodynamics. Nevertheless, many natural systems and processes will always remain far beyond the scope o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-equilibrium Thermodynamics
Non-equilibrium thermodynamics is a branch of thermodynamics that deals with physical systems that are not in thermodynamic equilibrium but can be described in terms of macroscopic quantities (non-equilibrium state variables) that represent an extrapolation of the variables used to specify the system in thermodynamic equilibrium. Non-equilibrium thermodynamics is concerned with transport processes and with the rates of chemical reactions. Almost all systems found in nature are not in thermodynamic equilibrium, for they are changing or can be triggered to change over time, and are continuously and discontinuously subject to flux of matter and energy to and from other systems and to chemical reactions. Some systems and processes are, however, in a useful sense, near enough to thermodynamic equilibrium to allow description with useful accuracy by currently known non-equilibrium thermodynamics. Nevertheless, many natural systems and processes will always remain far beyond the scope o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MaxEnt School
In physics, maximum entropy thermodynamics (colloquially, ''MaxEnt'' thermodynamics) views equilibrium thermodynamics and statistical mechanics as inference processes. More specifically, MaxEnt applies inference techniques rooted in Shannon information theory, Bayesian probability, and the principle of maximum entropy. These techniques are relevant to any situation requiring prediction from incomplete or insufficient data (e.g., image reconstruction, signal processing, spectral analysis, and inverse problems). MaxEnt thermodynamics began with two papers by Edwin T. Jaynes published in the 1957 ''Physical Review''. Maximum Shannon entropy Central to the MaxEnt thesis is the principle of maximum entropy. It demands as given some partly specified model and some specified data related to the model. It selects a preferred probability distribution to represent the model. The given data state "testable information" about the probability distribution, for example particular expectat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clausius
Rudolf Julius Emanuel Clausius (; 2 January 1822 – 24 August 1888) was a German physicist and mathematician and is considered one of the central founding fathers of the science of thermodynamics. By his restatement of Sadi Carnot's principle known as the Carnot cycle, he gave the theory of heat a truer and sounder basis. His most important paper, "On the Moving Force of Heat", published in 1850, first stated the basic ideas of the second law of thermodynamics. In 1865 he introduced the concept of entropy. In 1870 he introduced the virial theorem, which applied to heat. Life Clausius was born in Köslin (now Koszalin, Poland) in the Province of Pomerania in Prussia. His father was a Protestant pastor and school inspector, and Rudolf studied in the school of his father. In 1838, he went to the Gymnasium in Stettin. Clausius graduated from the University of Berlin in 1844 where he had studied mathematics and physics since 1840 with, among others, Gustav Magnus, Peter Gustav L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann's Constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constant, and in Planck's law of black-body radiation and Boltzmann's entropy formula, and is used in calculating thermal noise in resistors. The Boltzmann constant has dimensions of energy divided by temperature, the same as entropy. It is named after the Austrian scientist Ludwig Boltzmann. As part of the 2019 redefinition of SI base units, the Boltzmann constant is one of the seven " defining constants" that have been given exact definitions. They are used in various combinations to define the seven SI base units. The Boltzmann constant is defined to be exactly . Roles of the Boltzmann constant Macroscopically, the ideal gas law states that, for an ideal gas, the product of pressure and volume is proportional to the product of amount of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Information Entropy
In information theory, the entropy of a random variable is the average level of "information", "surprise", or "uncertainty" inherent to the variable's possible outcomes. Given a discrete random variable X, which takes values in the alphabet \mathcal and is distributed according to p: \mathcal\to , 1/math>: \Eta(X) := -\sum_ p(x) \log p(x) = \mathbb \log p(X), where \Sigma denotes the sum over the variable's possible values. The choice of base for \log, the logarithm, varies for different applications. Base 2 gives the unit of bits (or " shannons"), while base ''e'' gives "natural units" nat, and base 10 gives units of "dits", "bans", or " hartleys". An equivalent definition of entropy is the expected value of the self-information of a variable. The concept of information entropy was introduced by Claude Shannon in his 1948 paper " A Mathematical Theory of Communication",PDF archived froherePDF archived frohere and is also referred to as Shannon entropy. Shannon's theory d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |