|
Metal-catalyzed σ-bond Rearrangement
The metal ion-catalyzed σ-bond rearrangement is a collection of chemical reactions that occur with highly strained organic compounds are treated with metal ions like Ag+, Rh(I), or Pd(II) based reagents.Michael B. Smith, Jerry March, March's Advanced Organic Chemistry, 5th Ed., John Wiley & Sons, Inc., 2001, p. 1459. +2ring openings are sometimes observed: : These rearrangements proceed via oxidative addition of strained rings. Such processes are related to the activation of cyclopropanes by transition metals. See also * Cyclobutane * Cubane * Cuneane Cuneane () is a Saturated and unsaturated compounds, saturated hydrocarbon with the Chemical formula, formula and a Molecular geometry, 3D structure resembling a wedge, hence the name. Cuneane may be produced from cubane by metal-ion-catalyzed � ... * Sigma bond metathesis References {{DEFAULTSORT:Metal-ion-catalyzed sigma-bond rearrangement Ring expansion reactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
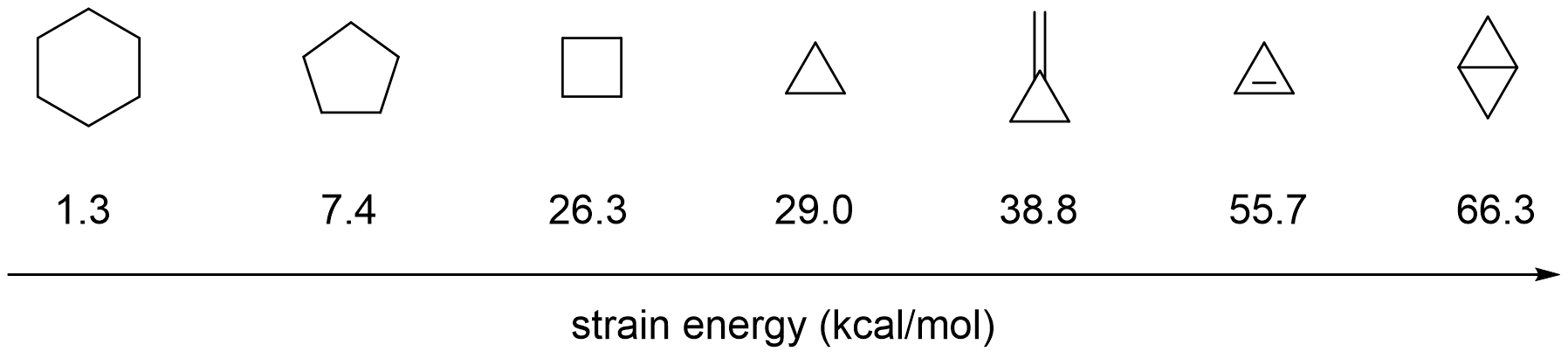
Ring Strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are substantially smaller than the idealized value of approximately 109°. Because of their high strain, the heat of combustion for these small rings is elevated. Ring strain results from a combination of angle strain, conformational strain or Pitzer strain (torsional eclipsing interactions), and transannular strain, also known as van der Waals strain or Prelog strain. The simplest examples of angle strain are small cycloalkanes such as cyclopropane and cyclobutane. Ring strain energy can be attributed to the energy required for the distortion of bond and bond angles in order to close a ring. Ring strain energy is believed to be the cause of accelerated rates in altering ring reactions. Its interactions with traditional bond energi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rearrangement Reaction
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule, hence these reactions are usually intramolecular. In the example below, the substituent R moves from carbon atom 1 to carbon atom 2: :\underset\ce\ce\underset\ce\ce Intermolecular rearrangements also take place. A rearrangement is not well represented by simple and discrete electron transfers (represented by curved arrows in organic chemistry texts). The actual mechanism of alkyl groups moving, as in Wagner–Meerwein rearrangement, probably involves transfer of the moving alkyl group fluidly along a bond, not ionic bond-breaking and forming. In pericyclic reactions, explanation by orbital interactions give a better picture than simple discrete electron transfers. It is, nevertheless, possible to draw the curved ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activation Of Cyclopropanes By Transition Metals
In organometallic chemistry, the activation of cyclopropanes by transition metals is a research theme with implications for organic synthesis and homogeneous catalysis. Being highly strained, cyclopropanes are prone to oxidative addition to transition metal complexes. The resulting metallacycles are susceptible to a variety of reactions. These reactions are rare examples of C-C bond activation. The rarity of C-C activation processes has been attributed to Steric effects that protect C-C bonds. Furthermore, the directionality of C-C bonds as compared to C-H bonds makes orbital interaction with transition metals less favorable. Thermodynamically, C-C bond activation is more favored than C-H bond activation as the strength of a typical C-C bond is around 90 kcal per mole while the strength of a typical unactivated C-H bond is around 104 kcal per mole. Two main approaches achieve C-C bond activation using a transition metal. One strategy is to increase the ring strain and the other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutane
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and is commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commercial or biological significance, but more complex derivatives are important in biology and biotechnology. Structure The bond angles between carbon atoms are significantly strained and as such have lower bond energies than related linear or unstrained hydrocarbons, e.g. butane or cyclohexane. As such, cyclobutane is unstable above about 500 °C. The four carbon atoms in cyclobutane are not coplanar; instead, the ring typically adopts a folded or "puckered" conformation. This implies that the C-C-C angle is less than 90°. One of the carbon atoms makes a 25° angle with the plane formed by the other three carbons. In this way, some of the eclipsing interactions are reduced. The conformation is also known as a "butterfly". Equivalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubane
Cubane is a synthetic hydrocarbon compound with the Chemical formula, formula . It consists of eight carbon atoms arranged at the corners of a Cube (geometry), cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons and a member of the prismanes. It was first synthesized in 1964 by Philip Eaton and Thomas Cole. Before this work, Eaton believed that cubane would be impossible to synthesize due to the "required 90 degree molecular geometry, bond angles". The cubic shape requires the carbon atoms to adopt an unusually sharp 90° bonding angle, which would be highly strain (chemistry), strained as compared to the tetrahedral molecular geometry#Tetrahedral bond angle, 109.45° angle of a tetrahedral geometry, tetrahedral carbon. Once formed, cubane is quite kinetic stability, kinetically stable, due to a lack of readily available decomposition paths. It is the simplest hydrocarbon with octahedral symmetry. Havi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cuneane
Cuneane () is a Saturated and unsaturated compounds, saturated hydrocarbon with the Chemical formula, formula and a Molecular geometry, 3D structure resembling a wedge, hence the name. Cuneane may be produced from cubane by metal-ion-catalyzed σ-bond rearrangement. Similar reactions are known for () and bishomocubane (). : Molecular geometry The carbon atoms in the cuneane molecule form a hexahedron with Molecular symmetry, point group C2v. The cuneane molecule has three kinds of equivalent carbon atoms (A, B, C), which have also been confirmed by NMR. The molecular graph of the carbon skeleton of cuneane is a regular graph with non-equivalent groups of vertices, and so it is a very important test object for different algorithms of mathematical chemistry. : Derivatives Some cuneane derivatives have liquid crystal properties. References {{Reflist Polycyclic nonaromatic hydrocarbons Cyclopropanes Cyclobutanes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma Bond Metathesis
In organometallic chemistry, sigma-bond metathesis is a chemical reaction wherein a metal-ligand sigma bond undergoes metathesis (exchange of parts) with the sigma bond in some reagent. The reaction is illustrated by the exchange of lutetium(III) methyl complex with a hydrocarbon (R-H): :(C5Me5)2Lu-CH3 + R-H → (C5Me5)2Lu-R + CH4 This reactivity was first observed by Patricia Watson, a researcher at duPont.{{cite journal, author=Watson, Patricia, title=Methane exchange reactions of lanthanide and early-transition-metal methyl complexes, journal=Journal of the American Chemical Society, year=1983, volume=32, pages=6491–6493, doi=10.1021/ja00359a023 The reaction is mainly observed for complexes of metals with d0 configuration, e.g. complexes of Sc(III), Zr(IV), Nb(V), Ta(V), etc. f-Element complexes also participate, regardless of the number of f-electrons. The reaction is thought to proceed via cycloaddition. Indeed, the rate of the reaction is characterized by a highly n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |