|
Knudsen Effusion Cell
In crystal growth, a Knudsen cell is an effusion evaporator source for relatively low partial pressure elementary sources (e.g. Ga, Al, Hg, As). Because it is easy to control the temperature of the evaporating material in Knudsen cells, they are commonly used in molecular-beam epitaxy. Development The Knudsen effusion cell was developed by Martin Knudsen (1871-1949). A typical Knudsen cell contains a crucible (made of pyrolytic boron nitride, quartz, tungsten or graphite), heating filaments (often made of metal tantalum), water cooling system, heat shields, and an orifice shutter. Vapor pressure measurement The Knudsen cell is used to measure the vapor pressures of a solid with very low vapor pressure. Such a solid forms a vapor at low pressure by sublimation. The vapor slowly effuses through the pinhole, and the loss of mass is proportional to the vapor pressure and can be used to determine this pressure.Peter Atkins Peter William Atkins (born 10 August 1940) is an Engli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Growth
A crystal is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. Crystal growth is a major stage of a crystallization process, and consists of the addition of new atoms, ions, or polymer strings into the characteristic arrangement of the crystalline lattice. The growth typically follows an initial stage of either homogeneous or heterogeneous (surface catalyzed) nucleation, unless a "seed" crystal, purposely added to start the growth, was already present. The action of crystal growth yields a crystalline solid whose atoms or molecules are close packed, with fixed positions in space relative to each other. The crystalline state of matter is characterized by a distinct structural rigidity and very high resistance to deformation (i.e. changes of shape and/or volume). Most crystalline solids have high values both of Young's modulus and of the shear modulus of elasticity. This contras ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as ''tantalium'', it is named after Tantalus, a villain in Greek mythology. Tantalum is a very hard, ductile, lustrous, blue-gray transition metal that is highly corrosion-resistant. It is part of the refractory metals group, which are widely used as components of strong high-melting-point alloys. It is a group 5 element, along with vanadium and niobium, and it always occurs in geologic sources together with the chemically similar niobium, mainly in the mineral groups tantalite, columbite and coltan. The chemical inertness and very high melting point of tantalum make it valuable for laboratory and industrial equipment such as reaction vessels and vacuum furnaces. It is used in tantalum capacitors for electronic equipment such as computers. Tantalum is considered a technology-critical element by the European Commission. History Tantalum was discovered in Sweden in 1802 by Anders ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clausius–Clapeyron Relation
The Clausius–Clapeyron relation, named after Rudolf Clausius and Benoît Paul Émile Clapeyron, specifies the temperature dependence of pressure, most importantly vapor pressure, at a discontinuous phase transition between two phases of matter of a single constituent. Its relevance to meteorology and climatology is the increase of the water-holding capacity of the atmosphere by about 7% for every 1 °C (1.8 °F) rise in temperature. Definition On a pressure–temperature (''P''–''T'') diagram, the line separating the two phases is known as the coexistence curve. The Clapeyron relation gives the slope of the tangents to this curve. Mathematically, :\frac = \frac=\frac, where \mathrmP/\mathrmT is the slope of the tangent to the coexistence curve at any point, L is the specific latent heat, T is the temperature, \Delta v is the specific volume change of the phase transition, and \Delta s is the specific entropy change of the phase transition. The Clausius–Cla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Of Sublimation
In thermodynamics, the enthalpy of sublimation, or heat of sublimation, is the heat required to sublimate (change from solid to gas) one mole of a substance at a given combination of temperature and pressure, usually standard temperature and pressure (STP). It is equal to the cohesive energy of the solid. For elemental metals, it is also equal to the standard enthalpy of formation of the gaseous metal atoms. The heat of sublimation is usually expressed in kJ/mol, although the less customary kJ/kg is also encountered. Sublimation enthalpies See also * Heat * Sublimation (chemistry) * Phase transition In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states o ... * Clausius-Clapeyron equation References {{DEFAULTSORT:Enthalpy Of Sublimation Enthalpy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peter Atkins
Peter William Atkins (born 10 August 1940) is an English chemist and a Fellow of Lincoln College at the University of Oxford. He retired in 2007. He is a prolific writer of popular chemistry textbooks, including ''Physical Chemistry'', ''Inorganic Chemistry'', and ''Molecular Quantum Mechanics''. Atkins is also the author of a number of popular science books, including ''Atkins' Molecules'', ''Galileo's Finger: The Ten Great Ideas of Science'' and ''On Being''. Career Atkins left school ( Dr Challoner's Grammar School, Amersham) at fifteen and took a job at Monsanto as a laboratory assistant. He studied for A-levels by himself and gained a place, following a last-minute interview, at the University of Leicester. Atkins studied chemistry there, obtaining a BSc degree in chemistry, and a PhD degree in 1964 for research into electron spin resonance spectroscopy, and other aspects of theoretical chemistry. Atkins then took a postdoctoral position at UCLA as a Harkness Fellow o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sublimation (phase Transition)
Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point in its phase diagram, which corresponds to the lowest pressure at which the substance can exist as a liquid. The reverse process of sublimation is deposition or desublimation, in which a substance passes directly from a gas to a solid phase. Sublimation has also been used as a generic term to describe a solid-to-gas transition (sublimation) followed by a gas-to-solid transition (deposition). While vaporization from liquid to gas occurs as evaporation from the surface if it occurs below the boiling point of the liquid, and as boiling with formation of bubbles in the interior of the liquid if it occurs at the boiling point, there is no such distinction for the solid-to-gas transition which always occurs as sublimation from the surface. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapor Pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's evaporation rate. It relates to the tendency of particles to escape from the liquid (or a solid). A substance with a high vapor pressure at normal temperatures is often referred to as '' volatile''. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the kinetic energy of its molecules also increases. As the kinetic energy of the molecules increases, the number of molecules transitioning into a vapor also increases, thereby increasing the vapor pressure. The vapor pressure of any substance increases non-linearly with temperature accord ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Shield
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is also often used to refer to the thermal energy contained in a system as a component of its internal energy and that is reflected in the temperature of the system. For both uses of the term, heat is a form of energy. An example of formal vs. informal usage may be obtained from the right-hand photo, in which the metal bar is "conducting heat" from its hot end to its cold end, but if the metal bar is considered a thermodynamic system, then the energy flowing within the metal bar is called internal energy, not heat. The hot metal bar is also transferring heat to its surroundings, a correct statement for both the strict and loose meanings of ''heat''. Another example of informal usage is the term '' heat content'', used despite the fact that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Cooling
Cooling tower and water discharge of a nuclear power plant Water cooling is a method of heat removal from components and industrial equipment. Evaporative cooling using water is often more efficient than air cooling. Water is inexpensive and non-toxic; however, it can contain impurities and cause corrosion. Water cooling is commonly used for cooling automobile internal combustion engines and power stations. Water coolers utilising convective heat transfer are used inside high-end personal computers to lower the temperature of CPUs. Other uses include the cooling of lubricant oil in pumps; for cooling purposes in heat exchangers; for cooling buildings in HVAC and in chillers. Mechanism Advantages Water is inexpensive, non-toxic, and available over most of the earth's surface. Liquid cooling offers higher thermal conductivity than air cooling. Water has unusually high specific heat capacity among commonly available liquids at room temperature and atmospheric pressure allowi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
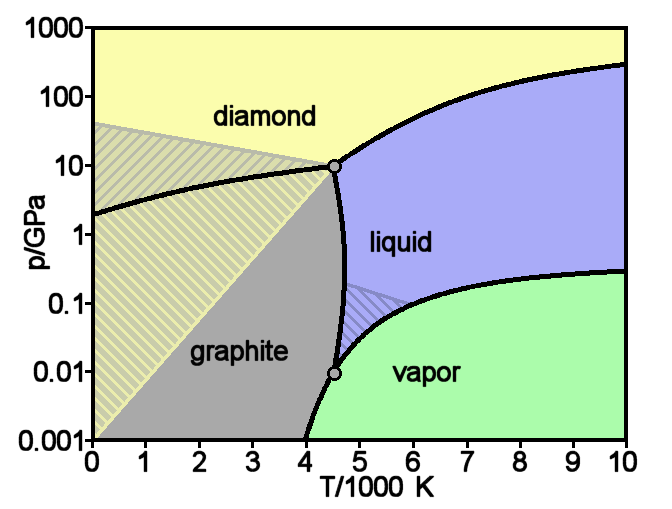
Graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large scale (300 kton/year, in 1989) for uses in pencils, lubricants, and electrodes. Under high pressures and temperatures it converts to diamond. It is a weak conductor of heat and electricity. Types and varieties Natural graphite The principal types of natural graphite, each occurring in different types of ore deposits, are * Crystalline small flakes of graphite (or flake graphite) occurs as isolated, flat, plate-like particles with hexagonal edges if unbroken. When broken the edges can be irregular or angular; * Amorphous graphite: very fine flake graphite is sometimes called amorphous; * Lump graphite (or vein graphite) occurs in fissure veins or fractures and appears as massive platy intergrowths of fibrous or acicular cryst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Effusion
In physics and chemistry, effusion is the process in which a gas escapes from a container through a hole of diameter considerably smaller than the mean free path of the molecules. Such a hole is often described as a ''pinhole'' and the escape of the gas is due to the pressure difference between the container and the exterior. Under these conditions, essentially all molecules which arrive at the hole continue and pass through the hole, since collisions between molecules in the region of the hole are negligible. Conversely, when the diameter is larger than the mean free path of the gas, flow obeys the Sampson flow law. In medical terminology, an effusion refers to accumulation of fluid in an anatomic space, usually without loculation. Specific examples include subdural, mastoid, pericardial and pleural effusions. Etymology The word effusion derives from the Latin word, effundo, which means "shed, pour forth, pour out, utter, lavish, waste." Effusion into vacuum Effusion fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |