|
Joule's Law
Joule effect and Joule's law are any of several different physical effects discovered or characterized by English physicist James Prescott Joule. These physical effects are not the same, but all are frequently or occasionally referred to in the literature as the "Joule effect" or "Joule law" These physical effects include: * "Joule's first law" (Joule heating), a physical law expressing the relationship between the heat generated and the current flowing through a conductor. * Joule's second law states that the internal energy of an ideal gas is independent of its volume and pressure, depending only on its temperature. * Magnetostriction, a property of ferromagnetic materials that causes them to change their shape when subjected to a magnetic field. * The Joule–Thomson effect (during Joule expansion), the temperature change of a gas (usually cooling) when it is allowed to expand freely. * The Gough–Joule effect or the Gow–Joule effect, which is the tendency of elastomers to c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
James Prescott Joule
James Prescott Joule (; 24 December 1818 11 October 1889) was an English physicist, mathematician and brewer, born in Salford, Lancashire. Joule studied the nature of heat, and discovered its relationship to mechanical work (see energy). This led to the law of conservation of energy, which in turn led to the development of the first law of thermodynamics. The SI derived unit of energy, the joule, is named after him. He worked with Lord Kelvin to develop an absolute thermodynamic temperature scale, which came to be called the Kelvin scale. Joule also made observations of magnetostriction, and he found the relationship between the current through a resistor and the heat dissipated, which is also called Joule's first law. His experiments about energy transformations were first published in 1843. Early years James Joule was born in 1818, the son of Benjamin Joule (1784–1858), a wealthy brewer, and his wife, Alice Prescott, on New Bailey Street in Salford. Joule was t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Joule's First Law
Joule heating, also known as resistive, resistance, or Ohmic heating, is the process by which the passage of an electric current through a conductor produces heat. Joule's first law (also just Joule's law), also known in countries of former USSR as the Joule–Lenz law,Джоуля — Ленца закон . ''Большая советская энциклопедия'', 3-е изд., гл. ред. А. М. Прохоров. Москва: Советская энциклопедия, 1972. Т. 8 () states that the of heating generated by an |
Internal Energy
The internal energy of a thermodynamic system is the total energy contained within it. It is the energy necessary to create or prepare the system in its given internal state, and includes the contributions of potential energy and internal kinetic energy. It keeps account of the gains and losses of energy of the system that are due to changes in its internal state. It does not include the kinetic energy of motion of the system as a whole, or any external energies from surrounding force fields. The internal energy of an isolated system is constant, which is expressed as the law of conservation of energy, a foundation of the first law of thermodynamics. The internal energy is an extensive property. The internal energy cannot be measured directly and knowledge of all its components is rarely interesting, such as the static rest mass energy of its constituent matter. Thermodynamics is chiefly concerned only with ''changes'' in the internal energy, not with its absolute value. Instea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ideal Gas
An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics. The requirement of zero interaction can often be relaxed if, for example, the interaction is perfectly elastic or regarded as point-like collisions. Under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules (or atoms for monatomic gas) play the role of the ideal particles. Many gases such as nitrogen, oxygen, hydrogen, noble gases, some heavier gases like carbon dioxide and mixtures such as air, can be treated as ideal gases within reasonable tolerances over a considerable parameter range around standard temperature and pressure. Generally, a gas behaves more like an ideal gas at higher temperature and lower pressu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetostriction
Magnetostriction (cf. electrostriction) is a property of magnetic materials that causes them to change their shape or dimensions during the process of magnetization. The variation of materials' magnetization due to the applied magnetic field changes the magnetostrictive strain until reaching its saturation value, λ. The effect was first identified in 1842 by James Joule when observing a sample of iron. This effect causes energy loss due to frictional heating in susceptible ferromagnetic cores. The effect is also responsible for the low-pitched humming sound that can be heard coming from transformers, where oscillating AC currents produce a changing magnetic field. Explanation Internally, ferromagnetic materials have a structure that is divided into '' domains'', each of which is a region of uniform magnetization. When a magnetic field is applied, the boundaries between the domains shift and the domains rotate; both of these effects cause a change in the material's dimensions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
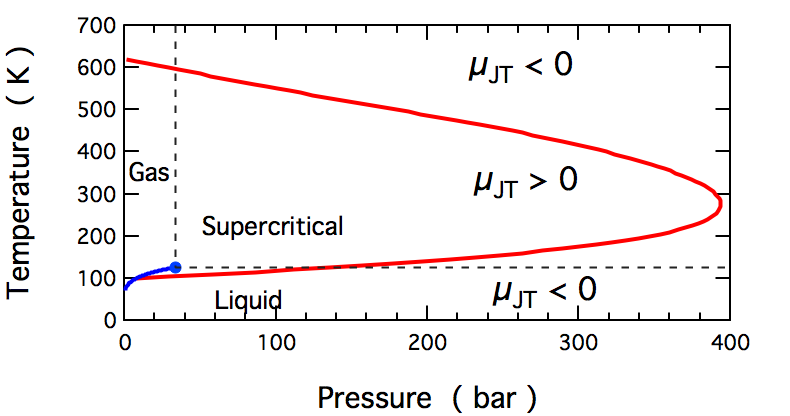
Joule–Thomson Effect
In thermodynamics, the Joule–Thomson effect (also known as the Joule–Kelvin effect or Kelvin–Joule effect) describes the temperature change of a ''real'' gas or liquid (as differentiated from an ideal gas) when it is forced through a valve or porous plug while keeping it insulated so that no heat is exchanged with the environment. This procedure is called a ''throttling process'' or ''Joule–Thomson process''. At room temperature, all gases except hydrogen, helium, and neon cool upon expansion by the Joule–Thomson process when being throttled through an orifice; these three gases experience the same effect but only at lower temperatures. Most liquids such as hydraulic oils will be warmed by the Joule–Thomson throttling process. The gas-cooling throttling process is commonly exploited in refrigeration processes such as liquefiers in air separation industrial process. In hydraulics, the warming effect from Joule–Thomson throttling can be used to find internall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
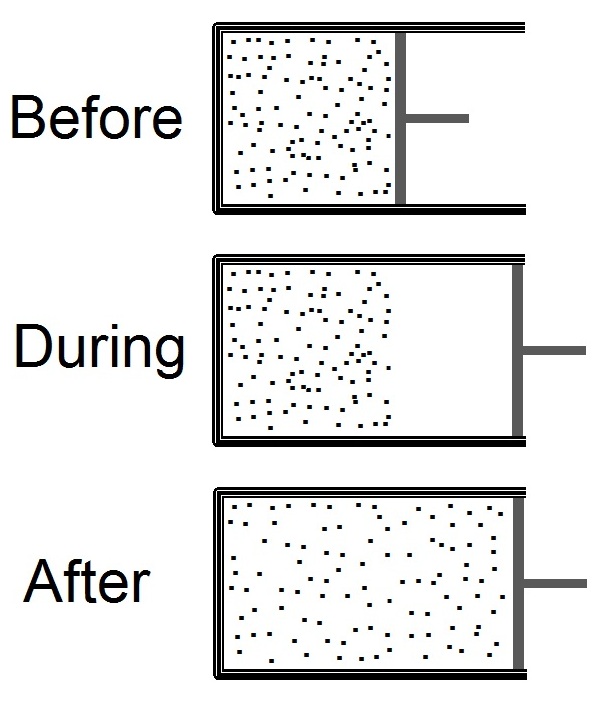
Joule Expansion
The Joule expansion (also called free expansion) is an irreversible process in thermodynamics in which a volume of gas is kept in one side of a thermally isolated container (via a small partition), with the other side of the container being evacuated. The partition between the two parts of the container is then opened, and the gas fills the whole container. The Joule expansion, treated as a thought experiment involving ideal gases, is a useful exercise in classical thermodynamics. It provides a convenient example for calculating changes in thermodynamic quantities, including the resulting increase in entropy of the universe (entropy production) that results from this inherently irreversible process. An actual Joule expansion experiment necessarily involves real gases; the temperature change in such a process provides a measure of intermolecular forces. This type of expansion is named after James Prescott Joule who used this expansion, in 1845, in his study for the mechanical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gough–Joule Effect
The Gough–Joule effect (a.k.a. Gow–Joule effect) is originally the tendency of elastomers to contract when heated if they are under tension. Elastomers that are not under tension do not see this effect. The term is also used more generally to refer to the dependence of the temperature of any solid on the mechanical deformation. This effect can be observed in nylon strings of classical guitars, whereby the string contracts as a result of heating. If an elastic band is first stretched and then subjected to heating, it will shrink rather than expand. This effect was first observed by John Gough in 1802, and was investigated further by James Joule in the 1850s, when it then became known as the Gough–Joule effect. ''Examples in Literature:'' * Popular Science magazine, January 1972: "A stretched piece of rubber ''contracts'' when heated. In doing so, it exerts a measurable increase in its pull. This surprising property of rubber was first observed by James Prescott Joule about ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elastomer
An elastomer is a polymer with viscoelasticity (i.e. both viscosity and Elasticity (physics), elasticity) and with weak intermolecular forces, generally low Young's modulus and high Deformation (mechanics), failure strain compared with other materials. The term, a portmanteau of ''elastic polymer'', is often used interchangeably with Synthetic rubber, rubber, although the latter is preferred when referring to vulcanization, vulcanisates. Each of the monomers which link to form the polymer is usually a compound of several chemical elements, elements among carbon, hydrogen, oxygen and silicon. Elastomers are amorphous polymer A polymer (; Greek '' poly-'', "many" + '' -mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...s maintained above their glass transition temperature, so that considerable segmental motion, molecular reconfo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Joule Heating
Joule heating, also known as resistive, resistance, or Ohmic heating, is the process by which the passage of an electric current through a conductor produces heat. Joule's first law (also just Joule's law), also known in countries of former USSR as the Joule–Lenz law, Assuming the element behaves as a perfect resistor and that the power is completely converted into heat, the formula can be re-written by substituting Ohm's law, V = I R , into the generalized power equation: P = IV = I^2R = V^2/R where ''R'' is the resistance. Alternating current When current varies, as it does in AC circuits, P(t) = U(t) I(t) where ''t'' is time and ''P'' is the instantaneous power being converted from electrical energy to heat. Far more often, the ''average'' power is of more interest than the instantaneous power: P_ = U_\text I_\text = I_\text^2 R = U_\text^2 / R where "avg" denotes average (mean) over one or more cycles, and "rms" denotes root mean square. These formulas are valid fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetostriction
Magnetostriction (cf. electrostriction) is a property of magnetic materials that causes them to change their shape or dimensions during the process of magnetization. The variation of materials' magnetization due to the applied magnetic field changes the magnetostrictive strain until reaching its saturation value, λ. The effect was first identified in 1842 by James Joule when observing a sample of iron. This effect causes energy loss due to frictional heating in susceptible ferromagnetic cores. The effect is also responsible for the low-pitched humming sound that can be heard coming from transformers, where oscillating AC currents produce a changing magnetic field. Explanation Internally, ferromagnetic materials have a structure that is divided into '' domains'', each of which is a region of uniform magnetization. When a magnetic field is applied, the boundaries between the domains shift and the domains rotate; both of these effects cause a change in the material's dimensions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |