|
Johannes Van Der Waals
Johannes Diderik van der Waals (; 23 November 1837 – 8 March 1923) was a Dutch theoretical physics, theoretical physicist and thermodynamicist famous for his pioneering work on the equation of state for gases and liquids. Van der Waals started his career as a school teacher. He became the first physics professor of the University of Amsterdam when in 1877 the old Athenaeum was upgraded to Municipal University. Van der Waals won the 1910 Nobel Prize in physics for his work on the equation of state for gases and liquids. His name is primarily associated with the Van der Waals equation of equation of state, state that describes the behavior of gases and their condensation to the liquid Phase (matter), phase. His name is also associated with Van der Waals forces (forces between stable molecules), with Van der Waals molecules (small molecular clusters bound by Van der Waals forces), and with Van der Waals radius, Van der Waals radii (sizes of molecules). As James Clerk Maxwell said ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leiden
Leiden (; in English and archaic Dutch also Leyden) is a city and municipality in the province of South Holland, Netherlands. The municipality of Leiden has a population of 119,713, but the city forms one densely connected agglomeration with its suburbs Oegstgeest, Leiderdorp, Voorschoten and Zoeterwoude with 206,647 inhabitants. The Netherlands Central Bureau of Statistics (CBS) further includes Katwijk in the agglomeration which makes the total population of the Leiden urban agglomeration 270,879, and in the larger Leiden urban area also Teylingen, Noordwijk, and Noordwijkerhout are included with in total 348,868 inhabitants. Leiden is located on the Oude Rijn, at a distance of some from The Hague to its south and some from Amsterdam to its north. The recreational area of the Kaag Lakes ( Kagerplassen) lies just to the northeast of Leiden. A university city since 1575, Leiden has been one of Europe's most prominent scientific centres for more than four centuries ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fritz London
Fritz Wolfgang London (March 7, 1900 – March 30, 1954) was a German physicist and professor at Duke University. His fundamental contributions to the theories of chemical bonding and of intermolecular forces ( London dispersion forces) are today considered classic and are discussed in standard textbooks of physical chemistry. With his brother Heinz London, he made a significant contribution to understanding electromagnetic properties of superconductors with the London equations and was nominated for the Nobel Prize in Chemistry on five separate occasions. Biography London was born in Breslau, Germany (now Wrocław, Poland) as the son of Franz London (1863-1917). Being a Jew, London lost his position at the University of Berlin after Hitler's Nazi Party passed the 1933 racial laws. He took visiting positions in England and France, and emigrated to the United States in 1939, of which he became a naturalized citizen in 1945. Later in his life, London was a professor at Duke Unive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Molecule
A Van der Waals molecule is a weakly bound complex of atoms or molecules held together by intermolecular attractions such as Van der Waals forces or by hydrogen bonds. The name originated in the beginning of the 1970s when stable molecular clusters were regularly observed in molecular beam microwave spectroscopy. Examples Examples of well-studied vdW molecules are Ar2, H2-Ar, H2O-Ar, benzene-Ar, (H2O)2, and (HF)2. Others include the largest diatomic molecule: He2 and LiHe. Supersonic beam spectroscopy In (supersonic) molecular beams temperatures are very low (usually less than 5 K). At these low temperatures Van der Waals (vdW) molecules are stable and can be investigated by microwave, far-infrared spectroscopy and other modes of spectroscopy. Also in cold equilibrium gases vdW molecules are formed, albeit in small, temperature dependent concentrations. Rotational and vibrational transitions in vdW molecules have been observed in gases, mainly by UV and IR spectrosco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Surface
The Van der Waals surface of a molecule is an abstract representation or model of that molecule, illustrating where, in very rough terms, a surface might reside for the molecule based on the hard cutoffs of Van der Waals radii for individual atoms, and it represents a surface through which the molecule might be conceived as interacting with other molecules. Also referred to as a ''Van der Waals envelope,'' the Van der Waals surface is named for Johannes Diderik van der Waals, a Dutch theoretical physicist and thermodynamicist who developed theory to provide a liquid-gas equation of state that accounted for the non-zero volume of atoms and molecules, and on their exhibiting an attractive force when they interacted (theoretical constructions that also bear his name). Van der Waals surfaces are therefore a tool used in the abstract representations of molecules, whether accessed, as they were originally, via hand calculation, or via physical wood/plastic models, or now digitally ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Radius
The van der Waals radius, ''r'', of an atom is the radius of an imaginary hard sphere representing the distance of closest approach for another atom. It is named after Johannes Diderik van der Waals, winner of the 1910 Nobel Prize in Physics, as he was the first to recognise that atoms were not simply points and to demonstrate the physical consequences of their size through the van der Waals equation of state. van der Waals volume The van der Waals volume, ''V'', also called the atomic volume or molecular volume, is the atomic property most directly related to the van der Waals radius. It is the volume "occupied" by an individual atom (or molecule). The van der Waals volume may be calculated if the van der Waals radii (and, for molecules, the inter-atomic distances, and angles) are known. For a single atom, it is the volume of a sphere whose radius is the van der Waals radius of the atom: V_ = \pi r_^3. For a molecule, it is the volume enclosed by the van der Waals surface ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Equation Of State
In chemistry and thermodynamics, the Van der Waals equation (or Van der Waals equation of state) is an equation of state which extends the ideal gas law to include the effects of interaction between molecules of a gas, as well as accounting for the finite size of the molecules. The ideal gas law treats gas molecules as point particles that interact with their containers but not each other, meaning they neither take up space nor change kinetic energy during collisions (i.e. all collisions are perfectly elastic). The ideal gas law states that the volume ''V'' occupied by ''n'' moles of any gas has a pressure ''P'' at temperature ''T'' given by the following relationship, where ''R'' is the gas constant: :PV=nRT To account for the volume occupied by real gas molecules, the Van der Waals equation replaces V/n in the ideal gas law with (V_m-b), where ''Vm'' is the molar volume of the gas and ''b'' is the volume occupied by the molecules of one mole: :P(V_m - b)=R T The seco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Forces
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and therefore more susceptible to disturbance. The van der Waals force quickly vanishes at longer distances between interacting molecules. Named after Dutch physicist Johannes Diderik van der Waals, the van der Waals force plays a fundamental role in fields as diverse as supramolecular chemistry, structural biology, polymer science, nanotechnology, surface science, and condensed matter physics. It also underlies many properties of organic compounds and molecular solids, including their solubility in polar and non-polar media. If no other force is present, the distance between atoms at which the force becomes repulsive rather than attractive as the atoms approach one another is called the van der Waals contact distance; this phenomenon result ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Real Gas Law
Real may refer to: Currencies * Brazilian real (R$) * Central American Republic real * Mexican real * Portuguese real * Spanish real * Spanish colonial real Music Albums * ''Real'' (L'Arc-en-Ciel album) (2000) * ''Real'' (Bright album) (2010) * ''Real'' (Belinda Carlisle album) (1993) * ''Real'' (Gorgon City EP) (2013) * ''Real'' (IU EP) (2010) * ''Real'' (Ivy Queen album) (2004) * ''Real'' (Mika Nakashima album) (2013) * ''Real'' (Ednita Nazario album) (2007) * ''Real'' (Jodie Resther album), a 2000 album by Jodie Resther * ''Real'' (Michael Sweet album) (1995) * ''Real'' (The Word Alive album) (2014) * ''Real'', a 2002 album by Israel Houghton recording as Israel & New Breed Songs * "Real" (Goo Goo Dolls song) (2008) * "Real" (Gorgon City song) (2013) * "Real" (Plumb song) (2004) * "Real" (Vivid song) (2012) * "Real" (James Wesley song) (2010) * "Real", a song by Kendrick Lamar from ''Good Kid, M.A.A.D City'' * "Real", a song by NF from ''Therapy Session'' * " ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Law Of Corresponding States
According to van der Waals, the theorem of corresponding states (or principle/law of corresponding states) indicates that all fluids, when compared at the same reduced temperature and reduced pressure, have approximately the same compressibility factor and all deviate from ideal gas behavior to about the same degree. Material constants that vary for each type of material are eliminated, in a recast reduced form of a constitutive equation. The reduced variables are defined in terms of critical variables. The principle originated with the work of Johannes Diderik van der Waals in about 1873 by Walter M. Kalback and Kenneth E. Starling, Chemical Engineering Department, |
Intermolecular Forces
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction or repulsion which act between atoms and other types of neighbouring particles, e.g. atoms or ions. Intermolecular forces are weak relative to intramolecular forces – the forces which hold a molecule together. For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than the forces present between neighboring molecules. Both sets of forces are essential parts of force fields frequently used in molecular mechanics. The investigation of intermolecular forces starts from macroscopic observations which indicate the existence and action of forces at a molecular level. These observations include non-ideal-gas thermodynamic behavior reflected by virial coefficients, vapor pressure, viscosity, superficial tension, and absorption data. The first reference to the nature of microscopic fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cryogenics
In physics, cryogenics is the production and behaviour of materials at very low temperatures. The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cryogenic” by accepting a threshold of 120 K (or –153 °C) to distinguish these terms from the conventional refrigeration. This is a logical dividing line, since the normal boiling points of the so-called permanent gases (such as helium, hydrogen, neon, nitrogen, oxygen, and normal air) lie below 120K while the Freon refrigerants, hydrocarbons, and other common refrigerants have boiling points above 120K. The U.S. National Institute of Standards and Technology considers the field of cryogenics as that involving temperatures below -153 Celsius (120K; -243.4 Fahrenheit) Discovery of superconducting materials with critical temperatures significantly above the boiling point of nitrogen has provided new interest in reliable, low cost meth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
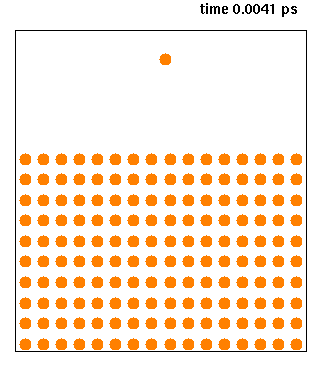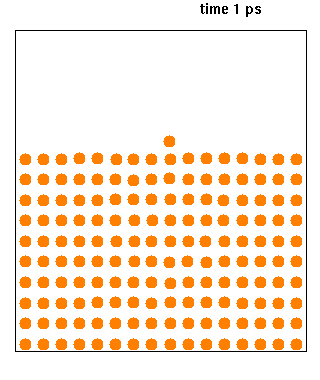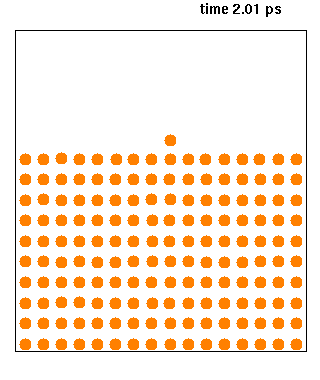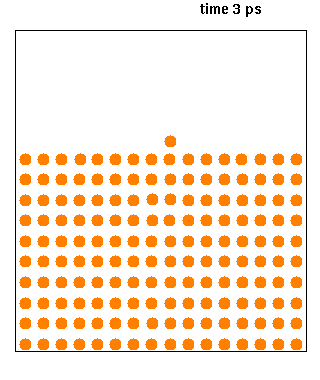
Molecular Dynamics
Molecular dynamics (MD) is a computer simulation method for analyzing the physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamic "evolution" of the system. In the most common version, the trajectories of atoms and molecules are determined by numerically solving Newton's equations of motion for a system of interacting particles, where forces between the particles and their potential energies are often calculated using interatomic potentials or molecular mechanical force fields. The method is applied mostly in chemical physics, materials science, and biophysics. Because molecular systems typically consist of a vast number of particles, it is impossible to determine the properties of such complex systems analytically; MD simulation circumvents this problem by using numerical methods. However, long MD simulations are mathematically ill-conditioned, generating cumulative erro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |