|
Janssen COVID-19 Vaccine
The Janssen COVID19 vaccine, sold under the brand name Jcovden, is a COVID19 vaccine that was developed by Janssen Vaccines in Leiden, Netherlands, and its Belgian parent company Janssen Pharmaceuticals, a subsidiary of American company Johnson & Johnson. It is a viral vector vaccine based on a human adenovirus that has been modified to contain the gene for making the spike protein of the SARS-CoV-2 virus that causes COVID19. The body's immune system responds to this spike protein to produce antibodies. The vaccine requires only one dose and does not need to be stored frozen. Clinical trials for the vaccine were started in June 2020, with phaseIII involving around 43,000 people. On 29 January 2021, Janssen announced that 28 days after a completed vaccination, the vaccine was 66% effective in a one-dose regimen in preventing symptomatic COVID19, with an 85% efficacy in preventing severe COVID19 and 100% efficacy in preventing hospitalization or death caused by the disease. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Severe Acute Respiratory Syndrome Coronavirus 2
Severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) is a strain of coronavirus that causes COVID-19 (coronavirus disease 2019), the respiratory illness responsible for the ongoing COVID-19 pandemic. The virus previously had a provisional name, 2019 novel coronavirus (2019-nCoV), and has also been called the human coronavirus 2019 (HCoV-19 or hCoV-19). First identified in the city of Wuhan, Hubei, China, the World Health Organization declared the outbreak a public health emergency of international concern on January 30, 2020, and a pandemic on March 11, 2020. SARS‑CoV‑2 is a positive-sense single-stranded RNA virus that is contagious in humans. SARS‑CoV‑2 is a virus of the species ''severe acute respiratory syndrome–related coronavirus'' (SARSr-CoV), related to the SARS-CoV-1 virus that caused the 2002–2004 SARS outbreak. Despite its close relation to SARS-CoV-1, its closest known relatives, with which it forms a sister group, are the derived SAR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viral Vector Vaccine
A viral vector vaccine is a vaccine that uses a viral vector to deliver genetic material ( DNA), which can be transcribed by the recipient's host cells as mRNA coding for a desired protein (or: antigen) to elicit an immune response. , six viral vector vaccines have been authorized for use in humans in at least one country: four COVID-19 vaccines and two Ebola vaccines. Technology Viral vector vaccines use a modified version of one virus as a vector to deliver to a cell a nucleic acid coding for an antigen for another infectious agent. Viral vector vaccines do not cause infection with either the virus used as the vector, or the source of the antigen. The genetic material it delivers does not integrate into a person's genome. Viral vector vaccines enable antigen expression within cells and induce a robust cytotoxic T cell response, unlike subunit vaccines which only confer humoral immunity. Most viral vectors are designed to be incapable of replication because the necessary gene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thrombosis With Thrombocytopenia Syndrome
Post-vaccination embolic and thrombotic events, termed vaccine-induced immune thrombotic thrombocytopenia (VITT), vaccine-induced prothrombotic immune thrombocytopenia (VIPIT), thrombosis with thrombocytopenia syndrome (TTS), vaccine-induced immune thrombocytopenia and thrombosis (VITT), or vaccine-associated thrombotic thrombocytopenia (VATT), are rare types of blood clotting syndromes that were initially observed in a number of people who had previously received the Oxford–AstraZeneca COVID‑19 vaccine (AZD1222) during the COVID‑19 pandemic. It was subsequently also described in the Janssen COVID‑19 vaccine (Johnson & Johnson) leading to suspension of its use until its safety had been reassessed. On 5 May 2022 the FDA posted a bulletin limiting the use of the Janssen Vaccine to very specific cases due to further reassesment of the risks of TTS, although the FDA also stated in the same bulletin that the benefits of the vaccine outweigh the risks. In April 2021, AstraZen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marketing Authorisation
Marketing authorisation is the process of reviewing and assessing the evidence to support a medicinal product, such as a drug, in relation to its marketing, finalised by granting of a licence to be sold. This process is performed within a legal framework defining the requirements necessary for successful application to the regulatory authority, details on the assessment procedure (based on quality, efficacy and safety criteria), and also the circumstances where a marketing authorisation already granted may be withdrawn, suspended or revoked. The application dossier for marketing authorisation is called a New Drug Application (NDA) in the USA or Marketing Authorisation Application (MAA) in the European Union and other countries, or simply registration dossier. This contains data proving that the drug has quality, efficacy and safety properties suitable for the intended use, additional administrative documents, samples of finished product or related substances and reagents necessar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emergency Use Authorization
An Emergency Use Authorization (EUA) in the United States is an authorization granted to the Food and Drug Administration (FDA) under sections of the Federal Food, Drug, and Cosmetic Act as added to and amended by various Acts of Congress, including by the Pandemic and All-Hazards Preparedness Reauthorization Act of 2013 (PAHPRA), as codified by , to allow the use of a drug prior to approval. It does not constitute ''approval'' of the drug in the full statutory meaning of the term, but instead authorizes the FDA to facilitate availability of an unapproved product, or an unapproved use of an approved product, during a declared state of emergency from one of several agencies or of a "material threat" by the Secretary of Homeland Security. Use EUAs have historically been infrequent. A review article by Rizk et al. provides a summary of the US experience in 2020 with pharmacological EUA approvals during the COVID-19 pandemic. It also provides a description of, and clinical rationale ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stat (website)
Stat (stylized STAT, sometimes also called Stat News) is an American health-oriented news website launched on November 4, 2015, by John W. Henry, the owner of ''The Boston Globe''. It is produced by Boston Globe Media and is headquartered in the ''Globe''s own building in Boston. Its executive editor is Rick Berke, who formerly worked at both ''The New York Times'' and ''Politico''. According to Kelsey Sutton of ''Politico'', the website is Henry's "biggest and most ambitious standalone site yet". The site's name comes from the term "stat", short for '' statim'', or "immediately"—a term that has long been used in medical contexts. As of February 2016, it had 45 staff members. Impact Notable stories Stat has broken include one about Robert Califf's research, published after then–President of the United States Barack Obama announced he would be his nominee to lead the Food and Drug Administration. The site also uncovered claims made by a vitamin company to which President Do ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase III Trials
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Summary Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory authority for use in the general population. Phase IV trials are 'post-marketing' or 'surveillance' studies conducted to monitor safety over sever ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michigan Medicine
Michigan Medicine (University of Michigan Health System or UMHS before 2017) is the wholly owned academic medical center of the University of Michigan, a public research university in Ann Arbor, Michigan. Michigan Medicine includes the University of Michigan Medical School, with its faculty group practice and research laboratories; the university's affiliated hospitals and health centers, including the University of Michigan Hospital, C.S. Mott Children's Hospital, Von Voigtlander Women's Hospital, and approximately 40 health centers and home care services across southeast Michigan; the clinical programs of the University of Michigan School of Nursing; and the activities of the Michigan Health Corporation, through which U-M partners with other medical centers and hospitals to provide specialized care throughout Michigan. History In 1869 the University of Michigan opened the first hospital in the country owned and operated by a university, in a house in Ann Arbor originally ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibody
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen. Each tip of the "Y" of an antibody contains a paratope (analogous to a lock) that is specific for one particular epitope (analogous to a key) on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can ''tag'' a microbe or an infected cell for attack by other parts of the immune system, or can neutralize it directly (for example, by blocking a part of a virus that is essential for its invasion). To allow the immune system to recognize millions of different antigens, the antigen-binding sites at both tips of the antibody come in an equally wide variety. In contrast, the remainder of the antibody is relatively constant. It only occurs in a few vari ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
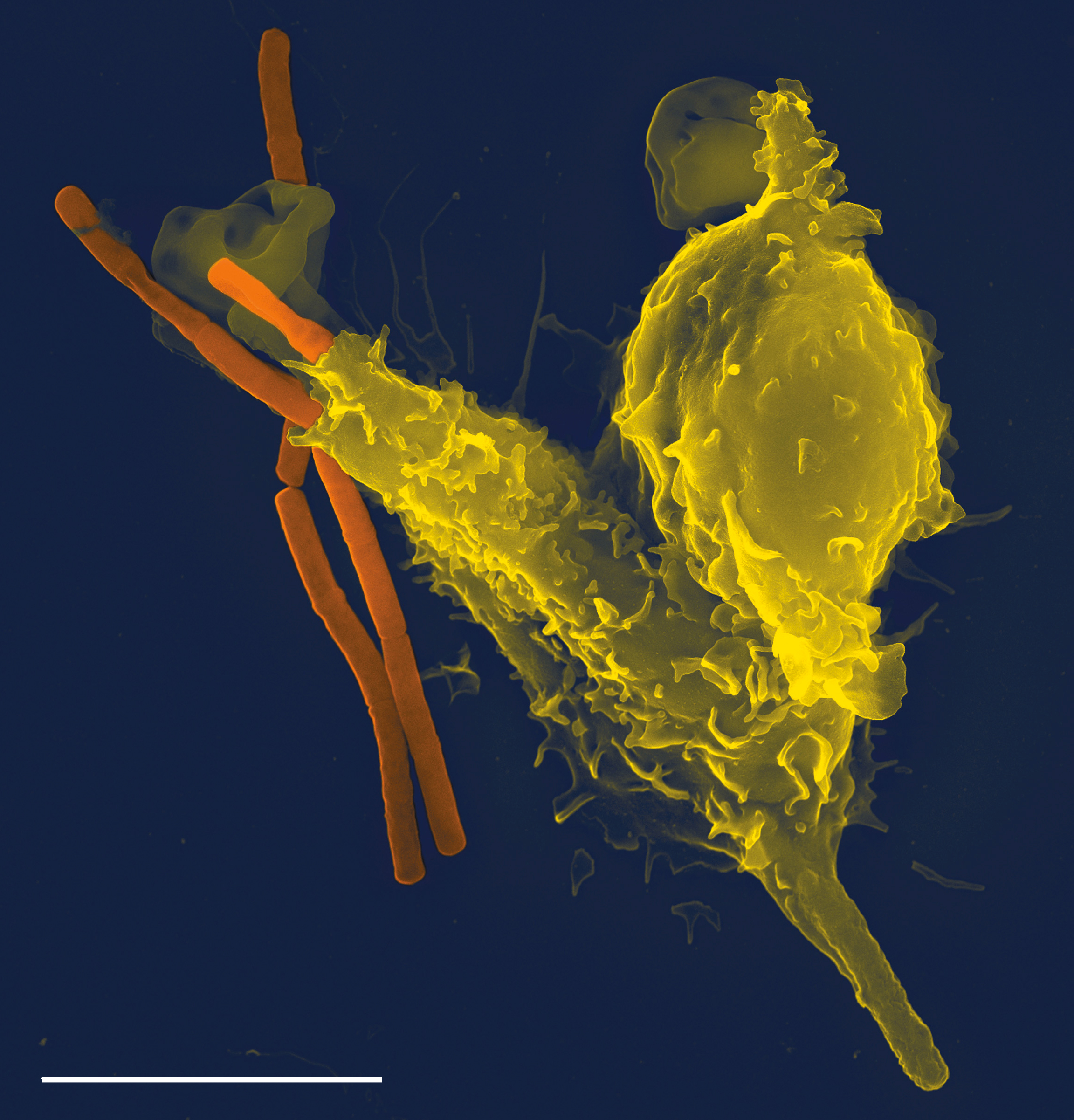
Immune System
The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splinters, distinguishing them from the organism's own healthy tissue. Many species have two major subsystems of the immune system. The innate immune system provides a preconfigured response to broad groups of situations and stimuli. The adaptive immune system provides a tailored response to each stimulus by learning to recognize molecules it has previously encountered. Both use molecules and cells to perform their functions. Nearly all organisms have some kind of immune system. Bacteria have a rudimentary immune system in the form of enzymes that protect against virus infections. Other basic immune mechanisms evolved in ancient plants and animals and remain in their modern descendants. These mechanisms include phagocytosis, antimicrobial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)
.jpg)