|
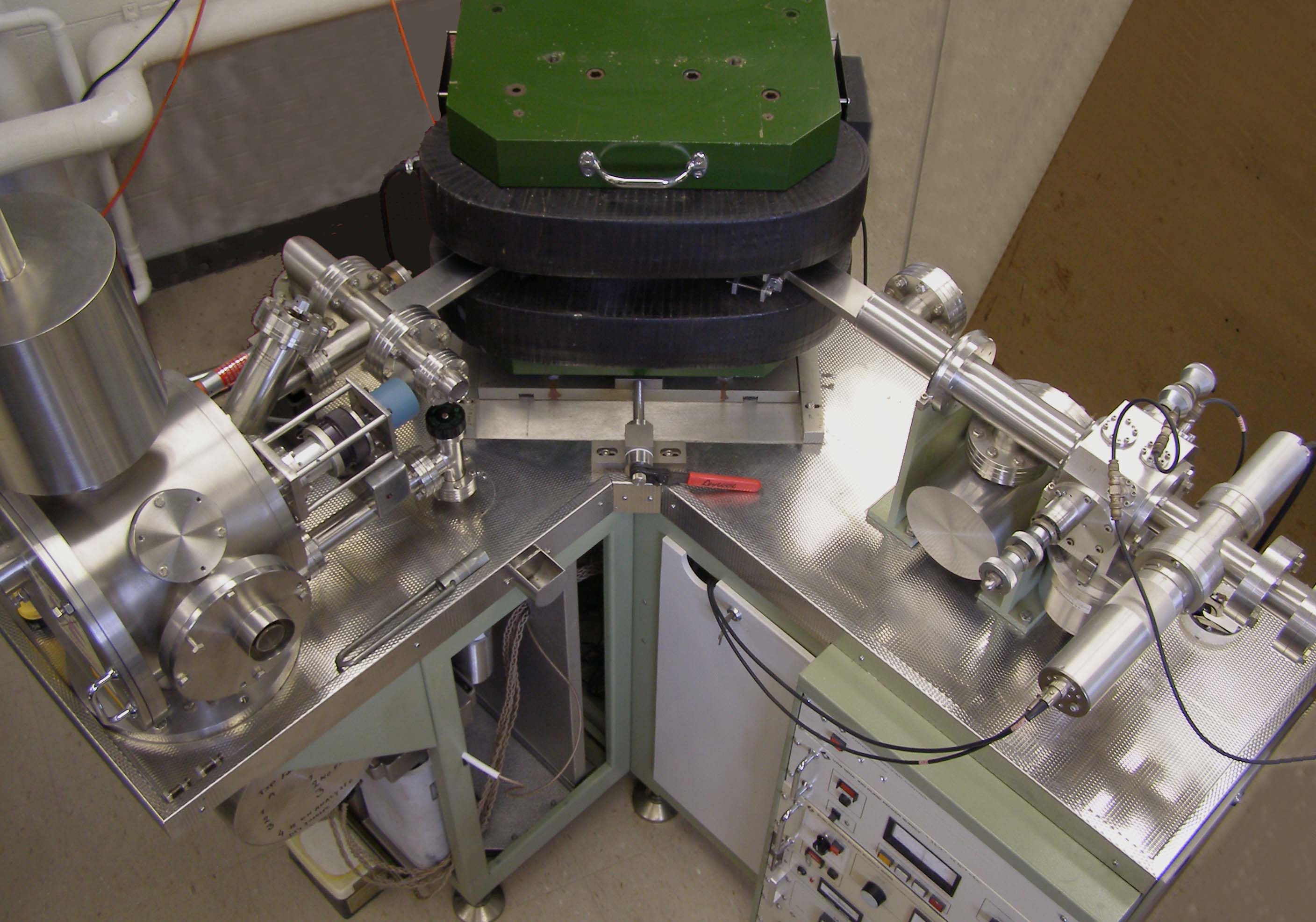
Isotope Ratio Mass Spectrometry
Isotope-ratio mass spectrometry (IRMS) is a specialization of mass spectrometry, in which mass spectrometric methods are used to measure the relative abundance of isotopes in a given sample. This technique has two different applications in the earth and environmental sciences. The analysis of 'stable isotopes' is normally concerned with measuring isotopic variations arising from mass-dependent isotopic fractionation in natural systems. On the other hand, radiogenic isotope analysis involves measuring the abundances of decay-products of natural radioactivity, and is used in most long-lived radiometric dating methods. Introduction The isotope-ratio mass spectrometer (IRMS) allows the precise measurement of mixtures of naturally occurring isotopes. Most instruments used for precise determination of isotope ratios are of the magnetic sector type. This type of analyzer is superior to the quadrupole type in this field of research for two reasons. First, it can be set up for multiple-co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Ionization
Thermal ionization, also known as surface ionization or contact ionization, is a physical process whereby the atoms are desorbed from a hot surface, and in the process are ionized. Thermal ionization is used to make simple ion sources, for mass spectrometry and for generating ion beams. Thermal ionization has seen extensive use in determining atomic weights, in addition to being used in many geological/nuclear applications. Physics The likelihood of ionization is a function of the filament temperature, the work function of the filament substrate and the ionization energy of the element. This is summarised in the Saha-Langmuir equation: :\frac = \frac \exp \Bigg(\frac\Bigg) where ::\frac = ratio of ion number density to neutral number density ::\frac = ratio of statistical weights (degeneracy) of ionic (g_+) and neutral (g_0) states ::W = work function of surface ::\Delta E_I = ionization energy of desorbed element ::k = Boltzmann's constant ::T = surface temperatu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium–lead Dating
Uranium–lead dating, abbreviated U–Pb dating, is one of the oldest and most refined of the radiometric dating schemes. It can be used to date rocks that formed and crystallised from about 1 million years to over 4.5 billion years ago with routine precisions in the 0.1–1 percent range. The method is usually applied to zircon. This mineral incorporates uranium and thorium atoms into its crystal structure, but strongly rejects lead when forming. As a result, newly-formed zircon deposits will contain no lead, meaning that any lead found in the mineral is radiogenic. Since the exact rate at which uranium decays into lead is known, the current ratio of lead to uranium in a sample of the mineral can be used to reliably determine its age. The method relies on two separate decay chains, the uranium series from 238U to 206Pb, with a half-life of 4.47 billion years and the actinium series from 235U to 207Pb, with a half-life of 710 million years. Decay routes Uranium decays to lead v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubidium–strontium Dating
The rubidium-strontium dating method is a radiometric dating technique, used by scientists to determine the age of rocks and minerals from their content of specific isotopes of rubidium (87Rb) and strontium (87Sr, 86Sr). One of the two naturally occurring isotopes of rubidium, 87Rb, decays to 87Sr with a half-life of 49.23 billion years. The radiogenic daughter, 87Sr, produced in this decay process is the only one of the four naturally occurring strontium isotopes that was not produced exclusively by stellar nucleosynthesis predating the formation of the Solar System. Over time, decay of 87Rb increases the amount of radiogenic 87Sr while the amount of other Sr isotopes remains unchanged. The ratio 87Sr/86Sr in a mineral sample can be accurately measured using a mass spectrometer. If the amount of Sr and Rb isotopes in the sample when it formed can be determined, the age can be calculated from the increase in 87Sr/86Sr. Different minerals that crystallized from the same silicic me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Ionization Mass Spectrometry
Thermal ionization mass spectrometry (TIMS) is also known as surface ionization and is a highly sensitive isotope mass spectrometry characterization technique. The isotopic ratios of radionuclides are used to get an accurate measurement for the elemental analysis of a sample. Singly charged ions of the sample are formed by the thermal ionization effect. A chemically purified liquid sample is placed on a metal filament which is then heated to evaporate the solvent. The removal of an electron from the purified sample is consequently achieved by heating the filament enough to release an electron, which then ionizes the atoms of the sample. TIMS utilizes a magnetic sector mass analyzer to separate the ions based on their mass to charge ratio. The ions gain velocity by an electrical potential gradient and are focused into a beam by electrostatic lenses. The ion beam then passes through the magnetic field of the electromagnet where it is partitioned into separate ion beams based on the io ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argon–argon Dating
Argon–argon (or 40Ar/39Ar) dating is a radiometric dating method invented to supersede potassiumargon (K/Ar) dating in accuracy. The older method required splitting samples into two for separate potassium and argon measurements, while the newer method requires only one rock fragment or mineral grain and uses a single measurement of argon isotopes. 40Ar/39Ar dating relies on neutron irradiation from a nuclear reactor to convert a stable form of potassium (39K) into the radioactive 39Ar. As long as a standard of known age is co-irradiated with unknown samples, it is possible to use a single measurement of argon isotopes to calculate the 40K/40Ar* ratio, and thus to calculate the age of the unknown sample. 40Ar* refers to the radiogenic 40Ar, i.e. the 40Ar produced from radioactive decay of 40K. 40Ar* does not include atmospheric argon adsorbed to the surface or inherited through diffusion and its calculated value is derived from measuring the 36Ar (which is assumed to be of atmosp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope Geochemistry
Isotope geochemistry is an aspect of geology based upon the study of natural variations in the relative abundances of isotopes of various elements. Variations in isotopic abundance are measured by isotope ratio mass spectrometry, and can reveal information about the ages and origins of rock, air or water bodies, or processes of mixing between them. Stable isotope geochemistry is largely concerned with isotopic variations arising from mass-dependent isotope fractionation, whereas radiogenic isotope geochemistry is concerned with the products of natural radioactivity. Stable isotope geochemistry For most stable isotopes, the magnitude of fractionation from kinetic and equilibrium fractionation is very small; for this reason, enrichments are typically reported in "per mil" (‰, parts per thousand). These enrichments (δ) represent the ratio of heavy isotope to light isotope in the sample over the ratio of a standard. That is, :\delta \ce = \left( \frac -1 \right) \times 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Noble Gases
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemical reactivity. The six naturally occurring noble gases are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn). Oganesson (Og) is a synthetically produced highly radioactive element. Although IUPAC has used the term "noble gas" interchangeably with "group 18" and thus included oganesson, it may not be significantly chemically noble and is predicted to break the trend and be reactive due to relativistic effects. Because of the extremely short 0.7 ms half-life of its only known isotope, its chemistry has not yet been investigated. For the first six periods of the periodic table, the noble gases are exactly the members of group 18. Noble gases are typically highly unreactive except whe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reference Materials For Stable Isotope Analysis
Isotopic reference materials are compounds (solids, liquids, gasses) with well-defined isotopic compositions and are the ultimate sources of accuracy in mass spectrometric measurements of isotope ratios. Isotopic references are used because mass spectrometers are highly fractionating. As a result, the isotopic ratio that the instrument measures can be very different from that in the sample's measurement. Moreover, the degree of instrument fractionation changes during measurement, often on a timescale shorter than the measurement's duration, and can depend on the characteristics of the sample itself. By measuring a material of known isotopic composition, fractionation within the mass spectrometer can be removed during post-measurement data processing. Without isotope references, measurements by mass spectrometry would be much less accurate and could not be used in comparisons across different analytical facilities. Due to their critical role in measuring isotope ratios, and in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope Ratio Ms
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties. The term isotope is formed from the Greek roots isos ( ἴσος "equal") and topos ( τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd in 1913 in a suggestion to the British chemist Frederick Soddy. The number of protons within the atom's nucleus is called its atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic nu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
South Carolina
)'' Animis opibusque parati'' ( for, , Latin, Prepared in mind and resources, links=no) , anthem = " Carolina";" South Carolina On My Mind" , Former = Province of South Carolina , seat = Columbia , LargestCity = Charleston , LargestMetro = Greenville (combined and metro) Columbia (urban) , BorderingStates = Georgia, North Carolina , OfficialLang = English , population_demonym = South Carolinian , Governor = , Lieutenant Governor = , Legislature = General Assembly , Upperhouse = Senate , Lowerhouse = House of Representatives , Judiciary = South Carolina Supreme Court , Senators = , Representative = 6 Republicans1 Democrat , postal_code = SC , TradAbbreviation = S.C. , area_rank = 40th , area_total_sq_mi = 32,020 , area_total_km2 = 82,932 , area_land_sq_mi = 30,109 , area_land_km2 = 77,982 , area_water_sq_mi = 1,911 , area_water_km2 = 4,949 , area_water_percent = 6 , population_rank = 23rd , population_as_of = 2022 , 2010Pop = 5282634 , populati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peedee Formation
The Peedee Formation is a geologic formation in North and South Carolina. A marine deposit, named for exposures along the Great Peedee River, it preserves belemnites and foraminifera fossils dating from the Late Cretaceous The Late Cretaceous (100.5–66 Ma) is the younger of two epochs into which the Cretaceous Period is divided in the geologic time scale. Rock strata from this epoch form the Upper Cretaceous Series. The Cretaceous is named after ''creta'', .... The formation is notable for its occurrence of '' Belemnitella americana'', known as the Pee Dee Belemnite (PDB), a long-standing standard in stable carbon isotope research. See also * List of fossiliferous stratigraphic units in South Carolina * List of fossiliferous stratigraphic units in North Carolina References External links * Cretaceous geology of North Carolina Cretaceous geology of South Carolina {{SouthCarolina-geologic-formation-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |