|
Isocitric Acid
Isocitric acid is a structural isomer of citric acid. Since citric acid and isocitric acid are structural isomers, they share similar physical and chemical properties. Due to these similar properties, it is difficult to separate the isomers. Salts and esters of isocitric acid are known as isocitrates. The isocitrate anion is a substrate of the citric acid cycle. Isocitrate is formed from citrate with the help of the enzyme aconitase, and is acted upon by isocitrate dehydrogenase. Isocitric acid is commonly used as a marker to detect the authenticity and quality of fruit products, most often citrus juices. In authentic orange juice, for example, the ratio of citric acid to D-isocitric acid is usually less than 130. An isocitric acid value higher than this may be indicative of fruit juice adulteration. Isocitric acid has largely been used as a biochemical agent due to limited amounts. However, isocitric acid has been shown to have pharmaceutical and therapeutic effects. Isocitric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Isomer
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct bonds between them. The term metamer was formerly used for the same concept. For example, butanol , methyl propyl ether , and diethyl ether have the same molecular formula but are three distinct structural isomers. The concept applies also to polyatomic ions with the same total charge. A classical example is the cyanate ion and the fulminate ion . It is also extended to ionic compounds, so that (for example) ammonium cyanate and urea are considered structural isomers,William F. Bynum, E. Janet Browne, Roy Porter (2014): ''Dictionary of the History of Science''. 530 pages. and so are methylammonium formate and ammonium acetate . Structural isomerism is the most radical type of isomerism. It is opposed to stereoisomerism, in which the atoms and bonding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parkinson's Disease
Parkinson's disease (PD), or simply Parkinson's, is a long-term degenerative disorder of the central nervous system that mainly affects the motor system. The symptoms usually emerge slowly, and as the disease worsens, non-motor symptoms become more common. The most obvious early symptoms are tremor, rigidity, slowness of movement, and difficulty with walking. Cognitive and behavioral problems may also occur with depression, anxiety, and apathy occurring in many people with PD. Parkinson's disease dementia becomes common in the advanced stages of the disease. Those with Parkinson's can also have problems with their sleep and sensory systems. The motor symptoms of the disease result from the death of cells in the substantia nigra, a region of the midbrain, leading to a dopamine deficit. The cause of this cell death is poorly understood, but involves the build-up of misfolded proteins into Lewy bodies in the neurons. Collectively, the main motor symptoms are also k ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tricarboxylic Acids
A tricarboxylic acid is an organic carboxylic acid whose chemical structure contains three carboxyl functional groups (-COOH). The best-known example of a tricarboxylic acid is citric acid. Uses Citric acid cycle Citric acid, a type of tricarboxylic acid, is used in the citric acid cycle – also known as tricarboxylic acid (TCA) cycle or Krebs cycle – which is fundamental to all aerobic organisms. Examples See also * Citric acid cycle (tricarboxylic acid cycle) * Dicarboxylic acid * Mellitic acid Mellitic acid, also called graphitic acid or benzenehexacarboxylic acid, is an acid first discovered in 1799 by Martin Heinrich Klaproth in the mineral mellite (honeystone), which is the aluminium salt of the acid. It crystallizes in fine silky ne ... Literature *{{cite journal , title = The Tricarboxylic Acid Cycle, an Ancient Metabolic Network with a Novel Twist. , author = Ryan J. Mailloux, Robin Bériault, Joseph Lemire, Ranji Singh, Daniel R. Chénier, Robert D. Hamel, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Hydroxy Acids
α-Hydroxy acids, or alpha hydroxy acids (AHAs), are a class of chemical compounds that consist of a carboxylic acid with a hydroxyl group substituent on the adjacent (alpha) carbon. Prominent examples are glycolic acid, lactic acid, mandelic acid and citric acid. Although these compounds are related to the ordinary carboxylic acids and are therefore weak acids, their chemical structure allows for the formation of an internal hydrogen bond between the hydrogen at the hydroxyl group and one of the oxygen atoms of the carboxylic group. The net effect is an increase in acidity. For example, the pKa of lactic acid is 3.86, while that of the unsubstituted propionic acid is 4.87; a full pKa unit difference means that lactic acid is ten times stronger than propionic acid. Industrial applications Feed additives 2-Hydroxy-4-(methylthio)butyric acid is produced commercially as a racemic mixture to substitute for methionine in animal feed. In nature, the same compound is an intermediate i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malic Acid
Malic acid is an organic compound with the molecular formula . It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms (L- and D-enantiomers), though only the L-isomer exists naturally. The salts and esters of malic acid are known as malates. The malate anion is an intermediate in the citric acid cycle. Etymology The word 'malic' is derived from Latin ' mālum', meaning 'apple'. The related Latin word , meaning 'apple tree', is used as the name of the genus ''Malus'', which includes all apples and crabapples; and the origin of other taxonomic classifications such as Maloideae, Malinae, and Maleae. Biochemistry L-Malic acid is the naturally occurring form, whereas a mixture of L- and D-malic acid is produced synthetically. File:L-Äpfelsäure.svg, L-Malic acid File:D-Äpfelsäure.svg, D-Malic acid Malate plays an important role in biochemistry. In the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tartaric Acid
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes, but also in bananas, tamarinds, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation. It is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E334 and to impart its distinctive sour taste. Naturally occurring tartaric acid is a useful raw material in organic chemical synthesis. Tartaric acid is an alpha-hydroxy-carboxylic acid, is diprotic and aldaric in acid characteristics, and is a dihydroxyl derivative of succinic acid. History Tartaric acid has been known to winemakers for centuries. However, the chemical process for extraction was developed in 1769 by the Swedish chemist Carl Wilhelm Scheele. Tartaric acid played an important role in the discovery of chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citric Acid
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms. More than two million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring, and a chelating agent. A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When part of a salt, the formula of the citrate anion is written as or . Natural occurrence and industrial production Citric acid occurs in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47 g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yarrowia Lipolytica
''Yarrowia'' is a fungal genus in the family Dipodascaceae. For a while the genus was monotypic, containing the single species ''Yarrowia lipolytica'', a yeast that can use unusual carbon sources, such as hydrocarbons. This has made it of interest for use in industrial microbiology, especially for the production of specialty lipids. Molecular phylogenetics analysis has revealed several other species that have since been added to the genus. In January 2019, ''Yarrowia lipolytica'' yeast biomass was defined by the European Food Safety Authority as a safe novel food – dried and heat‐killed – with the underlying qualifications that it is widespread in nature, present in the typical environment, may be used as food for people over age 3 (3 grams per day for children under age 10, and 6 grams per day for teens and adults), and may be manufactured as a dietary supplement. Biology Habitat ''Yarrowia lipolytica'' has been isolated from various locations (e.g. milled c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dextrorotation And Levorotation
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circular birefringence and circular dichroism are the manifestations of optical activity. Optical activity occurs only in chiral materials, those lacking microscopic mirror symmetry. Unlike other sources of birefringence which alter a beam's state of polarization, optical activity can be observed in fluids. This can include gases or solutions of chiral molecules such as sugars, molecules with helical secondary structure such as some proteins, and also chiral liquid crystals. It can also be observed in chiral solids such as certain crystals with a rotation between adjacent crystal planes (such as quartz) or metamaterials. When looking at the source of light, the rotation of the plane of polarization may be either to the right (dextrorotatory ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citric Acid
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms. More than two million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring, and a chelating agent. A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When part of a salt, the formula of the citrate anion is written as or . Natural occurrence and industrial production Citric acid occurs in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47 g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocitrate Dehydrogenase
Isocitrate dehydrogenase (IDH) () and () is an enzyme that catalyzes the oxidative decarboxylation of isocitrate, producing alpha-ketoglutarate (α-ketoglutarate) and CO2. This is a two-step process, which involves oxidation of isocitrate (a secondary alcohol) to oxalosuccinate (a ketone), followed by the decarboxylation of the carboxyl group beta to the ketone, forming alpha-ketoglutarate. In humans, IDH exists in three isoforms: IDH3 catalyzes the third step of the citric acid cycle while converting NAD+ to NADH in the mitochondria. The isoforms IDH1 and IDH2 catalyze the same reaction outside the context of the citric acid cycle and use NADP+ as a cofactor instead of NAD+. They localize to the cytosol as well as the mitochondrion and peroxisome. Isozymes The following is a list of human isocitrate dehydrogenase isozymes: NADP+ dependent Each NADP+-dependent isozyme functions as a homodimer: See also * Isocitrate/isopropylmalate dehydrogenase family NAD+ depen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
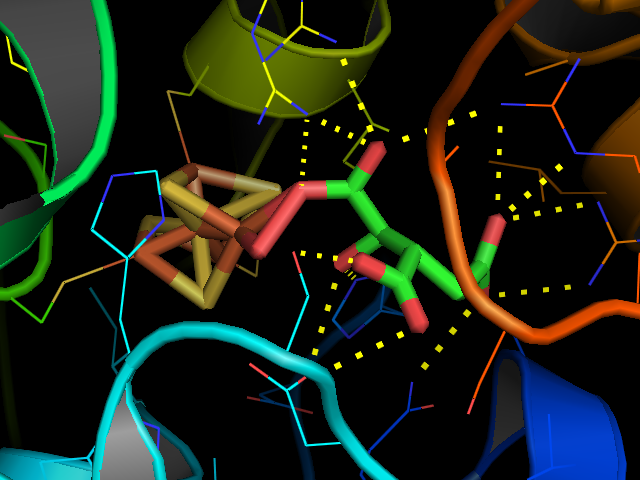
Aconitase
Aconitase (aconitate hydratase; ) is an enzyme that catalyses the stereo-specific isomerization of citrate to isocitrate via ''cis''-aconitate in the tricarboxylic acid cycle, a non- redox-active process. Image:Citrate wpmp.png, Image:Cis-Aconitate wpmp.png, Image:isocitric acid.svg, Structure Aconitase, displayed in the structures in the right margin of this page, has two slightly different structures, depending on whether it is activated or inactivated. In the inactive form, its structure is divided into four domains. Counting from the N-terminus, only the first three of these domains are involved in close interactions with the Fe-4Scluster, but the active site consists of residues from all four domains, including the larger C-terminal domain. The Fe-S cluster and a anion also reside in the active site. When the enzyme is activated, it gains an additional iron atom, creating a Fe-4Scluster. However, the structure of the rest of the enzyme is nearly unchanged; the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |