|
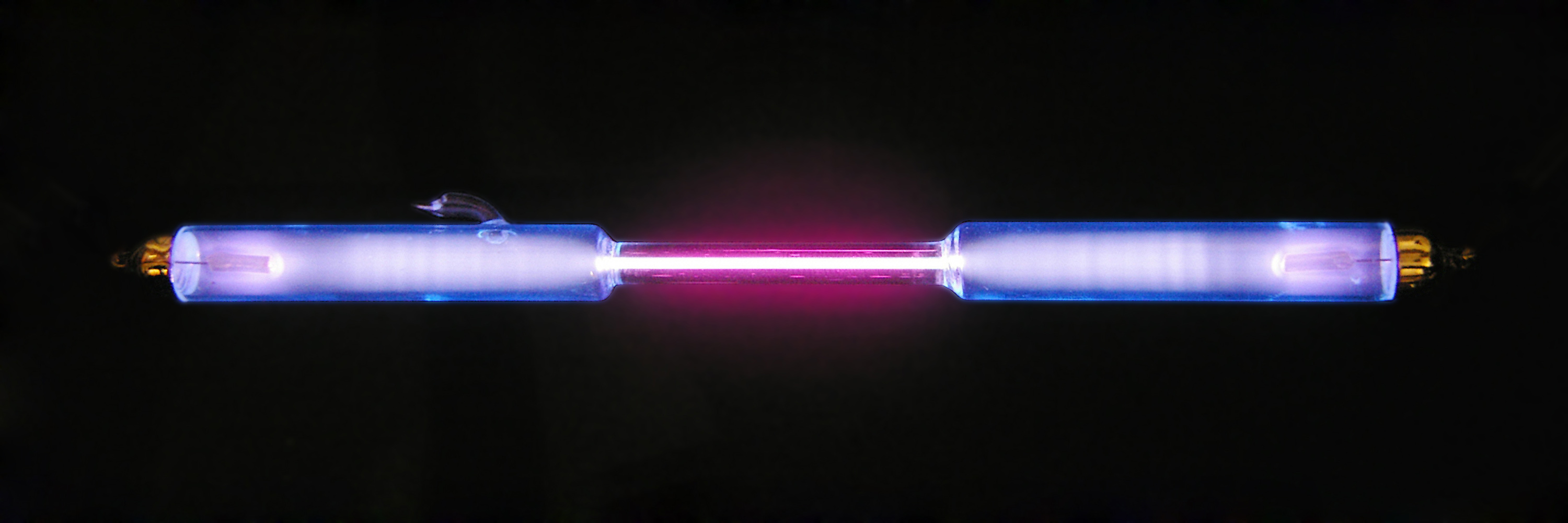
Iron Monohydride
Iron(I) hydride, systematically named iron hydride and poly(hydridoiron) is a solid inorganic compound with the chemical formula (also written or FeH). It is both thermodynamically and kinetically unstable toward decomposition at ambient temperature, and as such, little is known about its bulk properties. Iron(I) hydride is the simplest polymeric iron hydride. Due to its instability, it has no practical industrial uses. However, in metallurgical chemistry, iron(I) hydride is fundamental to certain forms of iron-hydrogen alloys. Nomenclature The systematic name ''iron hydride'', a valid IUPAC name, is constructed according to the compositional nomenclature. However, as the name is compositional in nature, it does not distinguish between compounds of the same stoichiometry, such as molecular species, which exhibit distinct chemical properties. The systematic names ''poly(hydridoiron)'' and ''poly errane(1)', also valid IUPAC names, are constructed according to the additive an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Hydride
An iron hydride is a chemical system which contains iron and hydrogen in some associated form. Because of the common occurrence of those two elements in the universe, possible compounds of hydrogen and iron have attracted attention. A few molecular compounds have been detected in extreme environments (such as stellar atmospheres) or detected in small amounts at very low temperatures. The two elements form a metallic alloy above of pressure, that has been advanced as a possible explanation for the low density of Earth's "iron" core. However those compounds are unstable when brought to ambient conditions, and eventually decompose into the separate elements. Small amounts of hydrogen (up to about 0.08% by weight) are absorbed into iron as it solidifies from molten state. Although the H2 is simply an impurity, its presence can affect the mechanical properties of the material. Despite the fleeting nature of binary iron hydrides, there are many fairly stable complexes containing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline Earth
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure. Structurally, they (together with helium) have in common an outer s-orbital which is full; that is, this orbital contains its full complement of two electrons, which the alkaline earth metals readily lose to form cations with charge +2, and an oxidation state of +2. All the discovered alkaline earth metals occur in nature, although radium occurs only through the decay chain of uranium and thorium and not as a primordial element. There have been experiments, all unsuccessful, to try to synthesize element 120, the next potential member of the group. Characteristics Chemical As with other groups, the members of this family show patterns in thei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can use d orbitals as valence orbitals to form chemical bonds. The lanthanide and actinide elements (the f-block) are called inner transition metals and are sometimes considered to be transition metals as well. Since they are metals, they are lustrous and have good electrical and thermal conductivity. Most (with the exception of group 11 and group 12) are hard and strong, and have high melting and boiling temperatures. They form compounds in any of two or more different oxidation states and bind to a variety of ligands to form coordination complexes that are often coloured. They form many useful alloys and are often employed as catalysts in elemental form or in compounds such as coordination complexes and oxides. Most are strongly paramag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sunspot
Sunspots are phenomena on the Sun's photosphere that appear as temporary spots that are darker than the surrounding areas. They are regions of reduced surface temperature caused by concentrations of magnetic flux that inhibit convection. Sunspots appear within active regions, usually in pairs of opposite magnetic polarity. Their number varies according to the approximately 11-year solar cycle. Individual sunspots or groups of sunspots may last anywhere from a few days to a few months, but eventually decay. Sunspots expand and contract as they move across the surface of the Sun, with diameters ranging from to . Larger sunspots can be visible from Earth without the aid of a telescope. They may travel at relative speeds, or proper motions, of a few hundred meters per second when they first emerge. Indicating intense magnetic activity, sunspots accompany other active region phenomena such as coronal loops, prominences, and reconnection events. Most solar flares and co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeeman Effect
The Zeeman effect (; ) is the effect of splitting of a spectral line into several components in the presence of a static magnetic field. It is named after the Dutch physicist Pieter Zeeman, who discovered it in 1896 and received a Nobel prize for this discovery. It is analogous to the Stark effect, the splitting of a spectral line into several components in the presence of an electric field. Also similar to the Stark effect, transitions between different components have, in general, different intensities, with some being entirely forbidden (in the dipole approximation), as governed by the selection rules. Since the distance between the Zeeman sub-levels is a function of magnetic field strength, this effect can be used to measure magnetic field strength, e.g. that of the Sun and other stars or in laboratory plasmas. The Zeeman effect is very important in applications such as nuclear magnetic resonance spectroscopy, electron spin resonance spectroscopy, magnetic resonance ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ground State
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. In quantum field theory, the ground state is usually called the vacuum state or the vacuum. If more than one ground state exists, they are said to be degenerate. Many systems have degenerate ground states. Degeneracy occurs whenever there exists a unitary operator that acts non-trivially on a ground state and commutes with the Hamiltonian of the system. According to the third law of thermodynamics, a system at absolute zero temperature exists in its ground state; thus, its entropy is determined by the degeneracy of the ground state. Many systems, such as a perfect crystal lattice, have a unique ground state and therefore have zero entropy at absolute zero. It is also possible for the highest excited state to have absolute zero te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons in the nucleus. Deuterium has a natural abundance in Earth's oceans of about one atom of deuterium among all atoms of hydrogen (see heavy water). Thus deuterium accounts for approximately 0.0156% by number (0.0312% by mass) of all the naturally occurring hydrogen in the oceans, while protium accounts for more than 99.98%. The abundance of deuterium changes slightly from one kind of natural water to another (see Vienna Standard Mean Ocean Water). ( Tritium is yet another hydrogen isotope, with two neutrons, that is far more rare and is radioactive.) The name ''deuterium'' is derived from the Greek , meaning "second", to denote the two particles composing the nucleus. De ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope Shift
The isotopic shift (also called isotope shift) is the shift in various forms of spectroscopy that occurs when one nuclear isotope is replaced by another. NMR spectroscopy In NMR spectroscopy, Isotopic effects on chemical shifts are typically small, far less than 1 ppm the typical unit for measuring shifts. The NMR signals for and ("HD") are readily distinguished in terms of their chemical shifts. The asymmetry of the signal for the "protio" impurity in arises from the differing chemical shifts of and . Vibrational spectra Isotopic shifts are best known and most widely used in vibration spectroscopy where the shifts are large, being proportional to the ratio of the square root of the isotopic masses. In the case of hydrogen, the "H-D shift" is (1/2)1/2 or 1/1.41. Thus, the (totally symmetric) C-H vibration for and occur at 2917 cm−1 and 2109 cm−1, respectively. This shift reflects the differing reduced mass for the affected bonds. Atomic spectra Iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Band Edge
Band or BAND may refer to: Places *Bánd, a village in Hungary *Band, Iran, a village in Urmia County, West Azerbaijan Province, Iran *Band, Mureș, a commune in Romania * Band-e Majid Khan, a village in Bukan County, West Azerbaijan Province, Iran People *Band (surname), various people with the surname Arts, entertainment, and media Music * Musical ensemble, a group of people who perform instrumental or vocal music **Band (rock and pop), a small ensemble that plays rock or pop ** Concert band, an ensemble of woodwind, brass, and percussion instruments **Dansband, band playing popular music for a partner-dancing audience ** Jazz band, a musical ensemble that plays jazz music **Marching band, a group of instrumental musicians who generally perform outdoors **School band, a group of student musicians who rehearse and perform instrumental music * The Band, a Canadian-American rock and roll group ** ''The Band'' (album), The Band's eponymous 1969 album * "Bands" (song), by American ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Near Infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around 1 millimeter (300 GHz) to the nominal red edge of the visible spectrum, around 700 nanometers (430 THz). Longer IR wavelengths (30 μm-100 μm) are sometimes included as part of the terahertz radiation range. Almost all black-body radiation from objects near room temperature is at infrared wavelengths. As a form of electromagnetic radiation, IR propagates energy and momentum, exerts radiation pressure, and has properties corresponding to both those of a wave and of a particle, the photon. It was long known that fires emit invisible heat; in 1681 the pioneering experimenter Edme Mariotte showed that glass, though transparent to sunlight, obstructed radiant heat. In 1800 the astronomer Sir William Herschel di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



_2007-04-30_T001456.gif)