|
Ideonella Sakaiensis
''Ideonella sakaiensis'' is a bacterium from the genus'' Ideonella'' and family Comamonadaceae capable of breaking down and consuming the plastic polyethylene terephthalate (PET) using it as both a carbon and energy source. The bacterium was originally isolated from a sediment sample taken outside of a plastic bottle recycling facility in Sakai City, Japan. Discovery ''Ideonella sakaiensis'' was first identified in 2016 by a team of researchers led by Kohei Oda of Kyoto Institute of Technology and Kenji Miyamoto of Keio University after collecting a sample of PET-contaminated sediment at a plastic bottle recycling facility in Sakai, Japan. The bacteria was first isolated from a consortium of microorganisms in the sediment sample, which included protozoa and yeast-like cells. The entire microbial community was shown to mineralize 75% of the degraded PET into carbon dioxide once it had been initially degraded and assimilated by ''Ideonella sakaiensis''. Characterization Physi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacterium
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit the air, soil, water, acidic hot springs, radioactive waste, and the deep biosphere of Earth's crust. Bacteria play a vital role in many stages of the nutrient cycle by recycling nutrients and the fixation of nitrogen from the atmosphere. The nutrient cycle includes the decomposition of dead bodies; bacteria are responsible for the putrefaction stage in this process. In the biological communities surrounding hydrothermal vents and cold seeps, extremophile bacteria provide the nutrients needed to sustain life by converting dissolved compounds, such as hydrogen sulphide and methane, to energy. Bacteria also live in mutualistic, commensal and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ideonella Sakaiensis Eating Plastics
''Ideonella'' is a genus of bacteria in the family Comamonadaceae. Applications ''Ideonella sakaiensis'' In 2016 ''I. sakaiensis'' was shown to degrade PET, a polymer widely used in food containers, bottles and synthetic fibers. Adhered to a low-grade PET film, the bacteria used two novel enzyme An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...s, PETase and MHETase, to decompose the plastic into two environmentally benign substances, which served as their main food source. A colony of ''I. sakaiensis'' could completely degrade a low-grade plastic water bottle in six weeks. Higher-grade PET products would require heating and cooling to weaken it before bacteria could start eating. The bacteria could also be used to reduce industrial waste during plastics manufacturing. Ref ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catechol
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is an organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amounts. It was first discovered by destructive distillation of the plant extract catechin. About 20,000 tonnes of catechol are now synthetically produced annually as a commodity organic chemical, mainly as a precursor to pesticides, flavors, and fragrances. Small amounts of catechol occur in fruits and vegetables. Isolation and synthesis Catechol was first isolated in 1839 by Edgar Hugo Emil Reinsch (1809–1884) by distilling it from the solid tannic preparation catechin, which is the residuum of catechu, the boiled or concentrated juice of ''Mimosa catechu'' ('' Acacia catechu''). Upon heating catechin above its decomposition point, a substance that Reinsch first named ''Brenz-Katechusäure'' (burned catechu acid) sublimated as a white efflo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-dihydroxy-3,5-cyclohexadiene-1,4-dicarboxylate Dehydrogenase
Onekama ( ) is a village in Manistee County in the U.S. state of Michigan. The population was 399 at the 2020 census. The village is located on the northeast shore of Portage Lake and is surrounded by Onekama Township. The town's name is derived from ''Ona-ga-maa'', an Anishinaabe word which means "singing water". History The predecessor of the village of Onekama was the settlement of Portage at Portage Point, first established in 1845, at the western end of Portage Lake, at the outlet of Portage Creek. In 1871, when landowners around the land-locked lake became exasperated with the practices of the Portage Sawmill, they took the solution into their own hands and dug a channel through the narrow isthmus, opening a waterway that lowered the lake by and brought it to the same level as Lake Michigan. When this action dried out Portage Creek on May 14, 1871, the settlement, which had only the week before been designated as "Onekama" with a post office under that name, moved to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
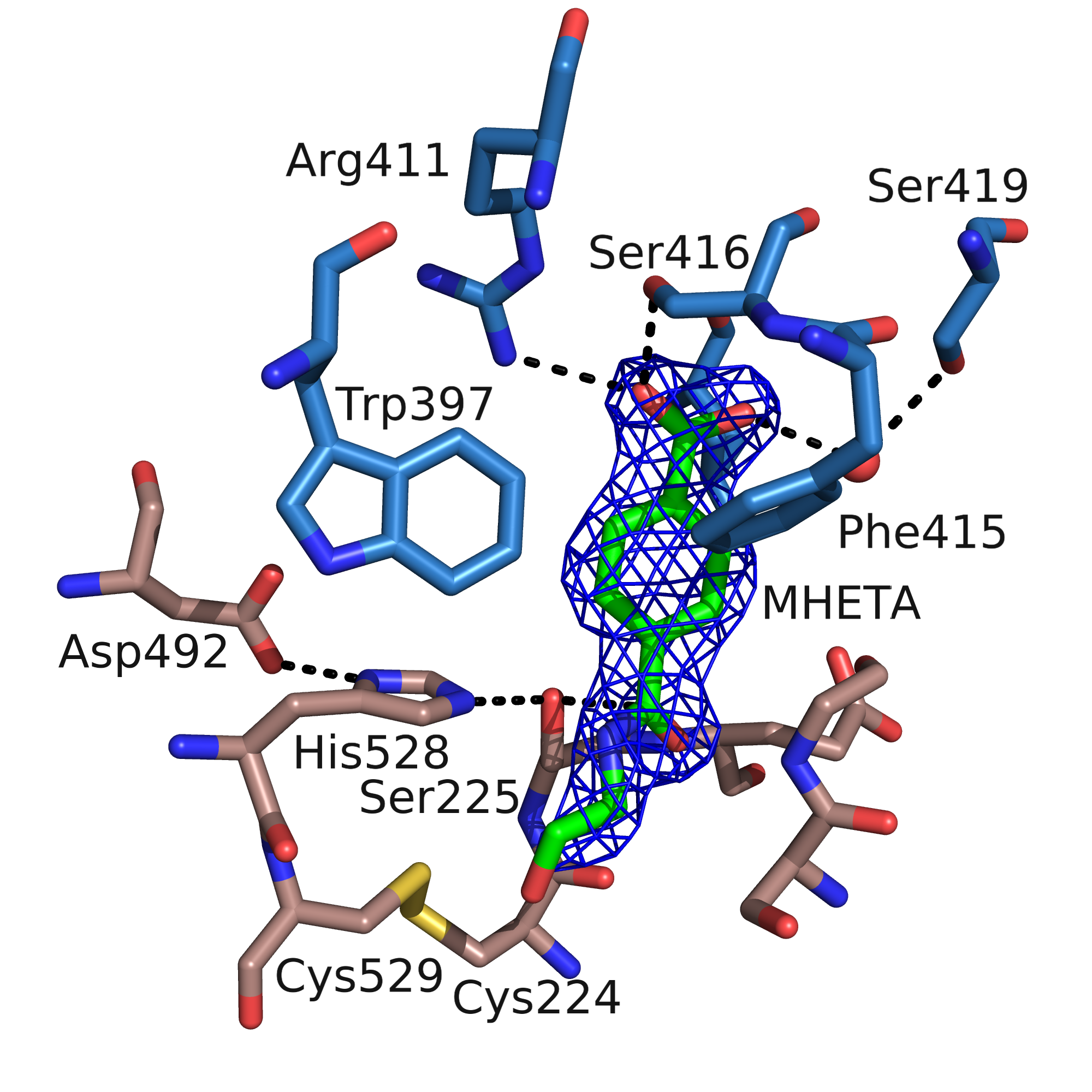
MHETase
The enzyme MHETase is a hydrolase, which was discovered in 2016. It cleaves 2-hydroxyethyl terephthalic acid, the PET degradation product by PETase, to ethylene glycol and terephthalic acid. This pair of enzymes, PETase and MHETase, enable the bacterium ''Ideonella sakaiensis'' to live on the plastic PET as sole carbon source. Chemical reaction The first enzyme of the PET degradation pathway, PETase, cleaves this plastic into the intermediates MHET ( Mono-(2-hydroxyethyl)terephthalic acid) and minor amounts BHET ( Bis-(2-hydroxyethyl)terephthalic acid). MHETase hydrolyses the ester bond of MHET forming terephthalic acid and ethylene glycol. Besides its natural substrate MHET the chromogenic substrate MpNPT, mono-''p''-nitrophenyl-terephthalate, is also hydrolyzed well. This can be used to measure the enzymatic activity and determine the kinetic parameters. Ferulate and gallate esters, substrates of the closest relatives in the tannase family, are not converted. ''p''-Nit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes: as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, flammable, viscous liquid. It has a sweet taste but is toxic in high concentrations. This molecule has been observed in outer space. Production Industrial routes Ethylene glycol is produced from ethylene (ethene), via the intermediate ethylene oxide. Ethylene oxide reacts with water to produce ethylene glycol according to the chemical equation : This reaction can be catalyzed by either acids or bases or can occur at neutral pH under elevated temperatures. The highest yields of ethylene glycol occur at acidic or neutral pH with a large excess of water. Under these conditions, ethylene glycol yields of 90% can be achieved. The major byproducts are the oligomers diethylene glycol, triethylene glycol, and tetra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terephthalic Acid
Terephthalic acid is an organic compound with formula C6H4(CO2H)2. This white solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tons are produced annually. The common name is derived from the turpentine-producing tree ''Pistacia terebinthus'' and phthalic acid. Terephthalic acid is also used in the production of PBT plastic (polybutylene terephthalate). History Terephthalic acid was first isolated (from turpentine) by the French chemist Amédée Cailliot (1805–1884) in 1846. Terephthalic acid became industrially important after World War II. Terephthalic acid was produced by oxidation of ''p''-xylene with 30-40% nitric acid. Air oxidation of ''p''-xylene gives ''p''-toluic acid, which resists further air-oxidation. Esterification of ''p''-toluic acid to methyl p-toluate (CH3C6H4CO2CH3) opens the way for further oxidation to monomethyl terephthalate. In the Dynamit−Nobel process t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterodimer
In biochemistry, a protein dimer is a macromolecular complex or multimer formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", '' di-'' + '' -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins while a protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein-coupled cannabinoid receptors have the ability to form both homo- and hetero ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Hydroxyethyl Terephthalic Acid
2-Hydroxyethyl terephthalic acid is an organic compound with the formula HOC2H4O2CC6H4CO2H. It is the monoester of terephthalic acid and ethylene glycol. The compound is a precursor to poly(ethylene terephthalate) (PET), a polymer that is produced on a large scale industrially. 2-Hydroxyethyl terephthalic acid is a colorless solid that is soluble in water and polar organic solvents. Near neutral pH, 2-hydroxyethyl terephthalic acid converts to 2-hydroxyethyl terephthalate, HOC2H4O2CC6H4CO2−. Occurrence and reactions 2-Hydroxyethyl terephthalic acid is an intermediate in both the formation and hydrolysis of PET. It is produced on a massive scale as the first intermediate in certain routes to PET. Specifically, it is produced in the course of the thermal condensation of terephthalic acid and ethylene glycol: :HOC2H4OH + HO2CC6H4CO2H → HOC2H4O2CC6H4CO2H + H2O Further dehydration of 2-hydroxyethyl terephthalic acid gives PET. It is also produced by the partial hydrolysis of PE ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PETase
PETases are an esterase class of enzymes that catalyze the breakdown (via hydrolysis) of polyethylene terephthalate (PET) plastic to monomeric mono-2-hydroxyethyl terephthalate (MHET). The idealized chemical reaction is: :(ethylene terephthalate)''n'' + H2O → (ethylene terephthalate)''n''-1 + MHET, where ''n'' is the number of monomers in the polymer chain, though a trace amount of the PET breaks down instead to bis(2-hydroxyethyl) terephthalate (BHET). PETases can also break down PEF-plastic ( polyethylene-2,5-furandicarboxylate), which is a bioderived PET replacement, into the analogous . PETases can't catalyze the hydrolysis of aliphatic polyesters like polybutylene succinate or polylactic acid. Whereas the degradation of PET by natural (non-enzymatic) means will take hundreds of years, PETases can degrade it in a matter of days. History The first PETase was discovered in 2016 from ''Ideonella sakaiensis'' strain 201-F6 bacteria found from sludge samples collected close ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolase
In biochemistry, hydrolases constitute a class of enzymes that commonly function as biochemical catalysts that use water to break a chemical bond: :\ce \quad \xrightarrowtext\quad \ce This typically results in dividing a larger molecule into smaller molecules. Some common examples of hydrolase enzymes are esterases including lipases, phosphatases, glycosidases, peptidases, and nucleosidases. Esterases cleave ester bonds in lipids and phosphatases cleave phosphate groups off molecules. An example of crucial esterase is acetylcholine esterase, which assists in transforming the neuron impulse into the acetate group after the hydrolase breaks the acetylcholine into choline and acetic acid. Acetic acid is an important metabolite in the body and a critical intermediate for other reactions such as glycolysis. Lipases hydrolyze glycerides. Glycosidases cleave sugar molecules off carbohydrates and peptidases hydrolyze peptide bonds. Nucleosidases hydrolyze the bonds of nucleo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |