|
Heat Capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K). Heat capacity is an extensive property. The corresponding intensive property is the specific heat capacity, found by dividing the heat capacity of an object by its mass. Dividing the heat capacity by the amount of substance in moles yields its molar heat capacity. The volumetric heat capacity measures the heat capacity per volume. In architecture and civil engineering, the heat capacity of a building is often referred to as its thermal mass. Definition Basic definition The heat capacity of an object, denoted by C, is the limit : C = \lim_\frac, where \Delta Q is the amount of heat that must be added to the object (of mass ''M'') in order to raise its temperature by \Delta T. The value of this parameter usually varies considerably dependin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Material Properties (thermodynamics)
The thermodynamic properties of materials are intensive thermodynamic parameters which are specific to a given material. Each is directly related to a second order differential of a thermodynamic potential. Examples for a simple 1-component system are: * Compressibility (or its inverse, the bulk modulus) :* Isothermal compressibility ::\kappa_T=-\frac\left(\frac\right)_T \quad = -\frac\,\frac :* Adiabatic compressibility ::\kappa_S=-\frac\left(\frac\right)_S \quad = -\frac\,\frac * Specific heat (Note - the extensive analog is the heat capacity) :* Specific heat at constant pressure ::c_P=\frac\left(\frac\right)_P \quad = -\frac\,\frac :* Specific heat at constant volume ::c_V=\frac\left(\frac\right)_V \quad = -\frac\,\frac * Coefficient of thermal expansion ::\alpha=\frac\left(\frac\right)_P \quad = \frac\,\frac where ''P'' is pressure, ''V'' is volume, ''T'' is temperature, ''S'' is entropy, and ''N'' is the number of particles. For a single comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Mass
In building design, thermal mass is a property of the mass of a building that enables it to store heat and provide inertia against temperature fluctuations. It is sometimes known as the thermal flywheel effect. The thermal mass of heavy structural elements can be designed to work alongside a construction's lighter thermal resistance components to create energy efficient buildings. For example, when outside temperatures are fluctuating throughout the day, a large thermal mass within the insulated portion of a house can serve to "flatten out" the daily temperature fluctuations, since the thermal mass will absorb thermal energy when the surroundings are higher in temperature than the mass, and give thermal energy back when the surroundings are cooler, without reaching thermal equilibrium. This is distinct from a material's insulative value, which reduces a building's thermal conductivity, allowing it to be heated or cooled relatively separately from the outside, or even just retain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per amount of substance, i.e. the pressure–volume product, rather than energy per temperature increment per ''particle''. The constant is also a combination of the constants from Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. It is a physical constant that is featured in many fundamental equations in the physical sciences, such as the ideal gas law, the Arrhenius equation, and the Nernst equation. The gas constant is the constant of proportionality that relates the energy scale in physics to the temperature scale and the scale used for amount of substance. Thus, the value of the gas constant ultimately derives from historical decisions and accidents in the setting of units of energy, temperature and amount of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mayer's Relation
In the 19th century, German chemist and physicist Julius von Mayer derived a relation between specific heat at constant pressure and the specific heat at constant volume for an ideal gas. Mayer's relation states that :C_ - C_ = R, where is the molar specific heat at constant pressure, is the molar specific heat at constant volume and is the gas constant. For more general homogeneous substances, not just ideal gases, the difference takes the form, :C_ - C_= V_ T\frac\, (see relations between heat capacities), where V_ is the molar volume, T is the temperature, \alpha_ is the thermal expansion coefficient and \beta is the isothermal compressibility. From this latter relation, several inferences can be made: * Since the isothermal compressibility \beta_ is positive for nearly all phases, and the square of thermal expansion coefficient is always either a positive quantity or zero, the specific heat at constant pressure is nearly always greater than or equal to specific heat a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isochoric Process
In thermodynamics, an isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant. An isochoric process is exemplified by the heating or the cooling of the contents of a sealed, inelastic container: The thermodynamic process is the addition or removal of heat; the isolation of the contents of the container establishes the closed system; and the inability of the container to deform imposes the constant-volume condition. The isochoric process here should be a quasi-static process. Formalism An isochoric thermodynamic quasi-static process is characterized by constant volume, i.e., . The process does no pressure-volume work, since such work is defined by W = P \Delta V , where is pressure. The sign convention is such that positive work is performed by the system on the environment. If the process is not quasi-stat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
First Law Of Thermodynamics
The first law of thermodynamics is a formulation of the law of conservation of energy, adapted for thermodynamic processes. It distinguishes in principle two forms of energy transfer, heat and thermodynamic work for a system of a constant amount of matter. The law also defines the internal energy of a system, an extensive property for taking account of the balance of energies in the system. The law of conservation of energy states that the total energy of any isolated system, which cannot exchange energy or matter, is constant. Energy can be transformed from one form to another, but can be neither created nor destroyed. The first law for a thermodynamic process is often formulated asThe sign convention (Q is heat supplied ''to'' the system but W is work done ''by'' the system) is that of Rudolf Clausius (Equation IIa on page 384 of Clausius, R. (1850)), and it is followed below. :\Delta U = Q - W, where \Delta U denotes the change in the internal energy of a closed syste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Internal Energy
The internal energy of a thermodynamic system is the total energy contained within it. It is the energy necessary to create or prepare the system in its given internal state, and includes the contributions of potential energy and internal kinetic energy. It keeps account of the gains and losses of energy of the system that are due to changes in its internal state. It does not include the kinetic energy of motion of the system as a whole, or any external energies from surrounding force fields. The internal energy of an isolated system is constant, which is expressed as the law of conservation of energy, a foundation of the first law of thermodynamics. The internal energy is an extensive property. The internal energy cannot be measured directly and knowledge of all its components is rarely interesting, such as the static rest mass energy of its constituent matter. Thermodynamics is chiefly concerned only with ''changes'' in the internal energy, not with its absolute value. Inste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Work (thermodynamics)
In thermodynamics, work is one of the principal processes by which a thermodynamic system can interact with its surroundings and exchange energy. An exchange of energy is facilitated by a mechanism through which the system can spontaneously exert macroscopic forces on its surroundings, or vice versa. In the surroundings, this mechanical work can lift a weight, for example. The externally measured forces and external effects may be electromagnetic,Guggenheim, E.A. (1985). ''Thermodynamics. An Advanced Treatment for Chemists and Physicists'', seventh edition, North Holland, Amsterdam, .Jackson, J.D. (1975). ''Classical Electrodynamics'', second edition, John Wiley and Sons, New York, .Konopinski, E.J. (1981). ''Electromagnetic Fields and Relativistic Particles'', McGraw-Hill, New York, . gravitational,North, G.R., Erukhimova, T.L. (2009). ''Atmospheric Thermodynamics. Elementary Physics and Chemistry'', Cambridge University Press, Cambridge (UK), . or mechanical (such as pressur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isobaric Process
In thermodynamics, an isobaric process is a type of thermodynamic process in which the pressure of the system stays constant: Δ''P'' = 0. The heat transferred to the system does work, but also changes the internal energy (''U'') of the system. This article uses the physics sign convention for work, where positive work is work done by the system. Using this convention, by the first law of thermodynamics, : Q = \Delta U + W\, where ''W'' is work, ''U'' is internal energy, and ''Q'' is heat. Pressure-volume work by the closed system is defined as: :W = \int \! p \,dV \, where Δ means change over the whole process, whereas ''d'' denotes a differential. Since pressure is constant, this means that : W = p \Delta V\, . Applying the ideal gas law, this becomes : W = n\,R\,\Delta T with ''R'' representing the gas constant, and ''n'' representing the amount of substance, which is assumed to remain constant (e.g., there is no phase transition during a chemica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pound (mass)
The pound or pound-mass is a unit of mass used in British imperial and United States customary systems of measurement. Various definitions have been used; the most common today is the international avoirdupois pound, which is legally defined as exactly , and which is divided into 16 avoirdupois ounces. The international standard symbol for the avoirdupois pound is lb; an alternative symbol is lbm (for most pound definitions), # ( chiefly in the U.S.), and or ″̶ (specifically for the apothecaries' pound). The unit is descended from the Roman (hence the abbreviation "lb"). The English word ''pound'' is cognate with, among others, German , Dutch , and Swedish . These units are historic and are no longer used (replaced by the metric system). Usage of the unqualified term ''pound'' reflects the historical conflation of mass and weight. This accounts for the modern distinguishing terms ''pound-mass'' and ''pound-force''. Etymology The word 'pound' and its cognate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust. In its metallic state, iron is rare in the Earth's crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kilns or furnaces capable of reaching or higher, about higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
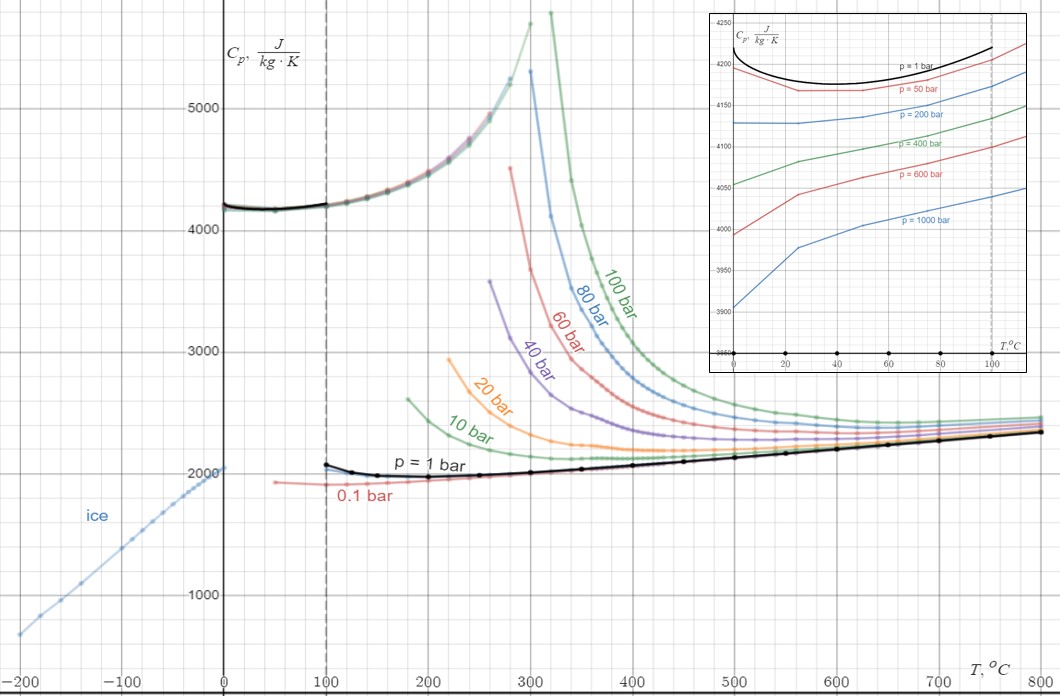
Heat Capacity Of Water 2
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is also often used to refer to the thermal energy contained in a system as a component of its internal energy and that is reflected in the temperature of the system. For both uses of the term, heat is a form of energy. An example of formal vs. informal usage may be obtained from the right-hand photo, in which the metal bar is "conducting heat" from its hot end to its cold end, but if the metal bar is considered a thermodynamic system, then the energy flowing within the metal bar is called internal energy, not heat. The hot metal bar is also transferring heat to its surroundings, a correct statement for both the strict and loose meanings of ''heat''. Another example of informal usage is the term '' heat content'', used despite the fact tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.png)

