|
Gas In A Box
In quantum mechanics, the results of the quantum particle in a box can be used to look at the equilibrium situation for a quantum ideal gas in a box which is a box containing a large number of molecules which do not interact with each other except for instantaneous thermalizing collisions. This simple model can be used to describe the classical ideal gas as well as the various quantum ideal gases such as the ideal massive Fermi gas, the ideal massive Bose gas as well as black body radiation (photon gas) which may be treated as a massless Bose gas, in which thermalization is usually assumed to be facilitated by the interaction of the photons with an equilibrated mass. Using the results from either Maxwell–Boltzmann statistics, Bose–Einstein statistics or Fermi–Dirac statistics, and considering the limit of a very large box, the Thomas–Fermi approximation (named after Enrico Fermi and Llewellyn Thomas) is used to express the degeneracy of the energy states as a differenti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, quantum field theory, quantum technology, and quantum information science. Classical physics, the collection of theories that existed before the advent of quantum mechanics, describes many aspects of nature at an ordinary (macroscopic) scale, but is not sufficient for describing them at small (atomic and subatomic) scales. Most theories in classical physics can be derived from quantum mechanics as an approximation valid at large (macroscopic) scale. Quantum mechanics differs from classical physics in that energy, momentum, angular momentum, and other quantities of a bound system are restricted to discrete values ( quantization); objects have characteristics of both particles and waves ( wave–particle duality); and there ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Particles In A Box
In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes a particle free to move in a small space surrounded by impenetrable barriers. The model is mainly used as a hypothetical example to illustrate the differences between classical and quantum systems. In classical systems, for example, a particle trapped inside a large box can move at any speed within the box and it is no more likely to be found at one position than another. However, when the well becomes very narrow (on the scale of a few nanometers), quantum effects become important. The particle may only occupy certain positive energy levels. Likewise, it can never have zero energy, meaning that the particle can never "sit still". Additionally, it is more likely to be found at certain positions than at others, depending on its energy level. The particle may never be detected at certain positions, known as spatial nodes. The particle in a box mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Planck's Law Of Black Body Radiation
In physics, Planck's law describes the spectral density of electromagnetic radiation emitted by a black body in thermal equilibrium at a given temperature , when there is no net flow of matter or energy between the body and its environment. At the end of the 19th century, physicists were unable to explain why the observed spectrum of black-body radiation, which by then had been accurately measured, diverged significantly at higher frequencies from that predicted by existing theories. In 1900, German physicist Max Planck heuristically derived a formula for the observed spectrum by assuming that a hypothetical electrically charged oscillator in a cavity that contained black-body radiation could only change its energy in a minimal increment, , that was proportional to the frequency of its associated electromagnetic wave. This resolved the problem of the ultraviolet catastrophe predicted by classical physics. This discovery was a pioneering insight of modern physics and is of fundam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Riemann Zeta Function
The Riemann zeta function or Euler–Riemann zeta function, denoted by the Greek letter (zeta), is a mathematical function of a complex variable defined as \zeta(s) = \sum_^\infty \frac = \frac + \frac + \frac + \cdots for \operatorname(s) > 1 and its analytic continuation elsewhere. The Riemann zeta function plays a pivotal role in analytic number theory, and has applications in physics, probability theory, and applied statistics. Leonhard Euler first introduced and studied the function over the reals in the first half of the eighteenth century. Bernhard Riemann's 1859 article " On the Number of Primes Less Than a Given Magnitude" extended the Euler definition to a complex variable, proved its meromorphic continuation and functional equation, and established a relation between its zeros and the distribution of prime numbers. This paper also contained the Riemann hypothesis, a conjecture about the distribution of complex zeros of the Riemann zeta function that is cons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polylogarithm
In mathematics, the polylogarithm (also known as Jonquière's function, for Alfred Jonquière) is a special function of order and argument . Only for special values of does the polylogarithm reduce to an elementary function such as the natural logarithm or a rational function. In quantum statistics, the polylogarithm function appears as the closed form of integrals of the Fermi–Dirac distribution and the Bose–Einstein distribution, and is also known as the Fermi–Dirac integral or the Bose–Einstein integral. In quantum electrodynamics, polylogarithms of positive integer order arise in the calculation of processes represented by higher-order Feynman diagrams. The polylogarithm function is equivalent to the Hurwitz zeta function — either function can be expressed in terms of the other — and both functions are special cases of the Lerch transcendent. Polylogarithms should not be confused with polylogarithmic functions nor with the offset logarithmic integral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Particle Number
The particle number (or number of particles) of a thermodynamic system, conventionally indicated with the letter ''N'', is the number of constituent particles in that system. The particle number is a fundamental parameter in thermodynamics which is conjugate to the chemical potential. Unlike most physical quantities, particle number is a dimensionless quantity. It is an extensive parameter, as it is directly proportional to the size of the system under consideration, and thus meaningful only for closed systems. A constituent particle is one that cannot be broken into smaller pieces at the scale of energy ''k·T'' involved in the process (where ''k'' is the Boltzmann constant and ''T'' is the temperature). For example, for a thermodynamic system consisting of a piston containing water vapour, the particle number is the number of water molecules in the system. The meaning of constituent particle, and thereby of particle number, is thus temperature-dependent. Determining the parti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maxwell–Boltzmann Distribution
In physics (in particular in statistical mechanics), the Maxwell–Boltzmann distribution, or Maxwell(ian) distribution, is a particular probability distribution named after James Clerk Maxwell and Ludwig Boltzmann. It was first defined and used for describing particle speeds in idealized gases, where the particles move freely inside a stationary container without interacting with one another, except for very brief collisions in which they exchange energy and momentum with each other or with their thermal environment. The term "particle" in this context refers to gaseous particles only ( atoms or molecules), and the system of particles is assumed to have reached thermodynamic equilibrium.''Statistical Physics'' (2nd Edition), F. Mandl, Manchester Physics, John Wiley & Sons, 2008, The energies of such particles follow what is known as Maxwell–Boltzmann statistics, and the statistical distribution of speeds is derived by equating particle energies with kinetic energy. Mathema ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Wavelength
In physics, the thermal de Broglie wavelength (\lambda_, sometimes also denoted by \Lambda) is roughly the average de Broglie wavelength of particles in an ideal gas at the specified temperature. We can take the average interparticle spacing in the gas to be approximately where is the volume and is the number of particles. When the thermal de Broglie wavelength is much smaller than the interparticle distance, the gas can be considered to be a classical or Maxwell–Boltzmann gas. On the other hand, when the thermal de Broglie wavelength is on the order of or larger than the interparticle distance, quantum effects will dominate and the gas must be treated as a Fermi gas or a Bose gas, depending on the nature of the gas particles. The critical temperature is the transition point between these two regimes, and at this critical temperature, the thermal wavelength will be approximately equal to the interparticle distance. That is, the quantum nature of the gas will be evident for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
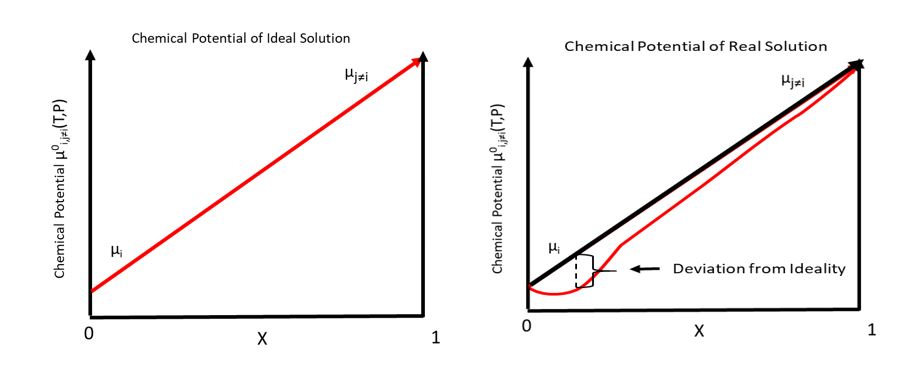
Chemical Potential
In thermodynamics, the chemical potential of a species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potential of a species in a mixture is defined as the rate of change of free energy of a thermodynamic system with respect to the change in the number of atoms or molecules of the species that are added to the system. Thus, it is the partial derivative of the free energy with respect to the amount of the species, all other species' concentrations in the mixture remaining constant. When both temperature and pressure are held constant, and the number of particles is expressed in moles, the chemical potential is the partial molar Gibbs free energy. At chemical equilibrium or in phase equilibrium, the total sum of the product of chemical potentials and stoichiometric coefficients is zero, as the free energy is at a minimum. In a system in diffusion equilibrium, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measurement, measured with a thermometer. Thermometers are calibrated in various Conversion of units of temperature, temperature scales that historically have relied on various reference points and thermometric substances for definition. The most common scales are the Celsius scale with the unit symbol °C (formerly called ''centigrade''), the Fahrenheit scale (°F), and the Kelvin scale (K), the latter being used predominantly for scientific purposes. The kelvin is one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale. Experimentally, it can be approached very closely but not actually reached, as recognized in the third law of thermodynamics. It would be impossible to extract energy as heat from a body at that temperature. Tem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann's Constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constant, and in Planck's law of black-body radiation and Boltzmann's entropy formula, and is used in calculating thermal noise in resistors. The Boltzmann constant has dimensions of energy divided by temperature, the same as entropy. It is named after the Austrian scientist Ludwig Boltzmann. As part of the 2019 redefinition of SI base units, the Boltzmann constant is one of the seven " defining constants" that have been given exact definitions. They are used in various combinations to define the seven SI base units. The Boltzmann constant is defined to be exactly . Roles of the Boltzmann constant Macroscopically, the ideal gas law states that, for an ideal gas, the product of pressure and volume is proportional to the product of amount of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Degenerate Energy Level
Degeneracy, degenerate, or degeneration may refer to: Arts and entertainment * ''Degenerate'' (album), a 2010 album by the British band Trigger the Bloodshed * Degenerate art, a term adopted in the 1920s by the Nazi Party in Germany to describe modern art ** Decadent movement, often associated with degeneracy * Dégénération, a single by Mylène Farmer * ''Degeneration'' (Nordau), an 1892 book by Max Nordau * '' Resident Evil: Degeneration'', a 2008 film * "Degenerate", a song by Blink-182 from the album ''Dude Ranch'' * "Degenerates", a song by A Day to Remember from the album '' You're Welcome'' Science, mathematics, and medicine Mathematics * Degeneracy (mathematics), a limiting case in which a class of object changes its nature so as to belong to another, usually simpler, class * Degeneracy (graph theory), a measure of the sparseness of a graph * Degeneration (algebraic geometry), the act of taking a limit of a family of varieties * Degenerate form, bilinear f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |