|
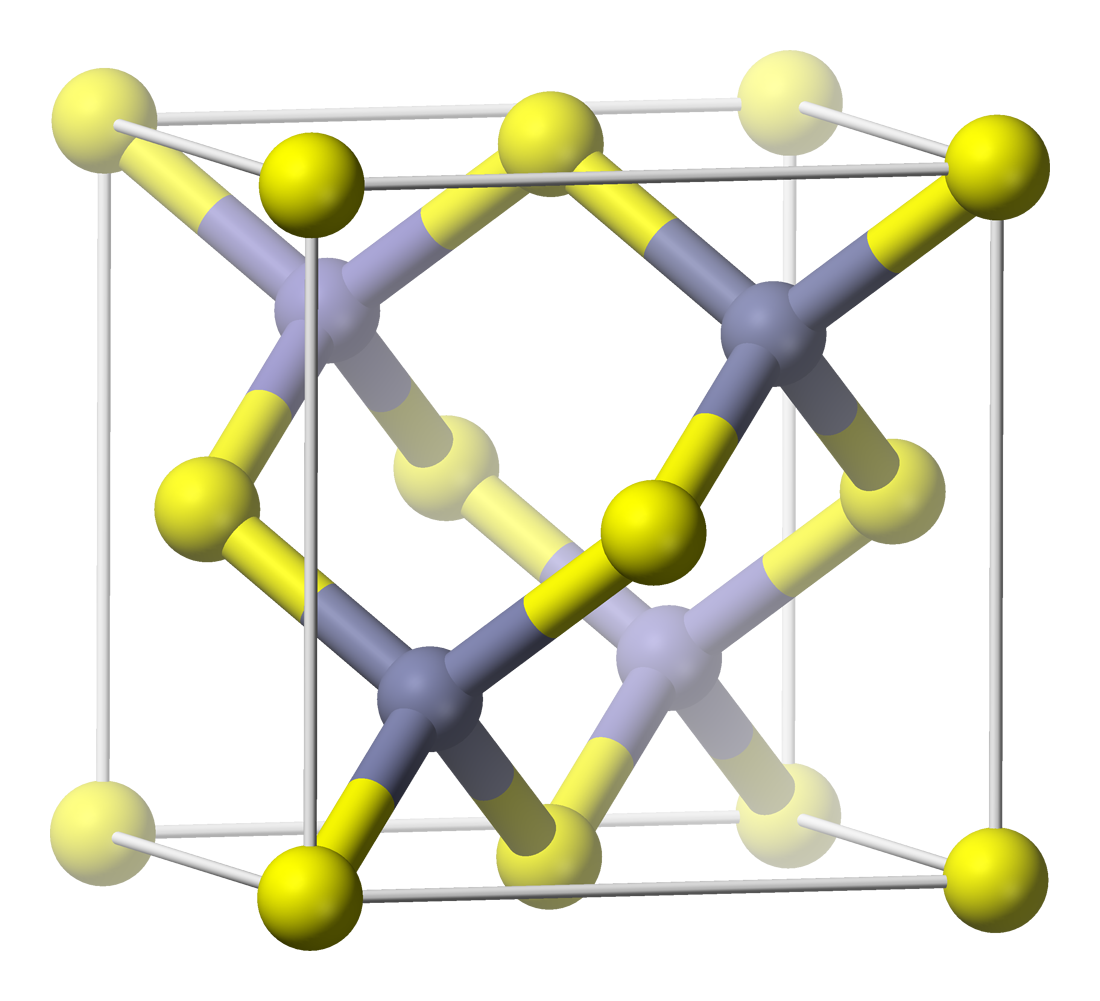
GaAs
Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a zinc blende crystal structure. Gallium arsenide is used in the manufacture of devices such as microwave frequency integrated circuits, monolithic microwave integrated circuits, infrared light-emitting diodes, laser diodes, solar cells and optical windows. GaAs is often used as a substrate material for the epitaxial growth of other III-V semiconductors, including indium gallium arsenide, aluminum gallium arsenide and others. Preparation and chemistry In the compound, gallium has a +3 oxidation state. Gallium arsenide single crystals can be prepared by three industrial processes: * The vertical gradient freeze (VGF) process. * Crystal growth using a horizontal zone furnace in the Bridgman-Stockbarger technique, in which gallium and arsenic vapors react, and free molecules deposit on a seed crystal at the cooler end of the furnace. * Liquid encapsulated Czochralski (LEC) growth is used for producing high-pu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium Gallium Arsenide
Indium gallium arsenide (InGaAs) (alternatively gallium indium arsenide, GaInAs) is a ternary alloy (chemical compound) of indium arsenide (InAs) and gallium arsenide (GaAs). Indium and gallium are ( group III) elements of the periodic table while arsenic is a (group V) element. Alloys made of these chemical groups are referred to as "III-V" compounds. InGaAs has properties intermediate between those of GaAs and InAs. InGaAs is a room-temperature semiconductor with applications in electronics and photonics. The principal importance of GaInAs is its application as a high-speed, high sensitivity photodetector of choice for optical fiber telecommunications. Nomenclature Indium gallium arsenide (InGaAs) and gallium-indium arsenide (GaInAs) are used interchangeably. According to IUPAC standards the preferred nomenclature for the alloy is GaxIn1-xAs where the group-III elements appear in order of increasing atomic number, as in the related alloy system AlxGa1-xAs. By far, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
III-V
Semiconductor materials are nominally small band gap insulators. The defining property of a semiconductor material is that it can be compromised by doping it with impurities that alter its electronic properties in a controllable way. Because of their application in the computer and photovoltaic industry—in devices such as transistors, lasers, and solar cells—the search for new semiconductor materials and the improvement of existing materials is an important field of study in materials science. Most commonly used semiconductor materials are crystalline inorganic solids. These materials are classified according to the periodic table groups of their constituent atoms. Different semiconductor materials differ in their properties. Thus, in comparison with silicon, compound semiconductors have both advantages and disadvantages. For example, gallium arsenide (GaAs) has six times higher electron mobility than silicon, which allows faster operation; wider band gap, which all ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar Cells
A solar cell, or photovoltaic cell, is an electronic device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon.Solar Cells chemistryexplained.com It is a form of photoelectric cell, defined as a device whose electrical characteristics, such as , , or resistance, vary when exposed to light. Individual solar cell devices are often the electrica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laser Diode
The laser diode chip removed and placed on the eye of a needle for scale A laser diode (LD, also injection laser diode or ILD, or diode laser) is a semiconductor device similar to a light-emitting diode in which a diode pumped directly with electrical current can create lasing conditions at the diode's junction. Driven by voltage, the doped p–n-transition allows for recombination of an electron with a hole. Due to the drop of the electron from a higher energy level to a lower one, radiation, in the form of an emitted photon is generated. This is spontaneous emission. Stimulated emission can be produced when the process is continued and further generates light with the same phase, coherence and wavelength. The choice of the semiconductor material determines the wavelength of the emitted beam, which in today's laser diodes range from infra-red to the UV spectrum. Laser diodes are the most common type of lasers produced, with a wide range of uses that include fiber optic c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Light-emitting Diode
A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (corresponding to the energy of the photons) is determined by the energy required for electrons to cross the band gap of the semiconductor. White light is obtained by using multiple semiconductors or a layer of light-emitting phosphor on the semiconductor device. Appearing as practical electronic components in 1962, the earliest LEDs emitted low-intensity infrared (IR) light. Infrared LEDs are used in remote-control circuits, such as those used with a wide variety of consumer electronics. The first visible-light LEDs were of low intensity and limited to red. Early LEDs were often used as indicator lamps, replacing small incandescent bulbs, and in seven-segment displays. Later developments produced LEDs available in visible, ultraviolet (U ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminum Gallium Arsenide
Aluminium gallium arsenide (also gallium aluminium arsenide) ( Alx Ga1−x As) is a semiconductor material with very nearly the same lattice constant as GaAs, but a larger bandgap. The ''x'' in the formula above is a number between 0 and 1 - this indicates an arbitrary alloy between GaAs and AlAs. The chemical formula ''AlGaAs'' should be considered an abbreviated form of the above, rather than any particular ratio. The bandgap varies between 1.42 eV (GaAs) and 2.16 eV (AlAs). For x < 0.4, the bandgap is direct. The is related with the bandgap via the Kramers–Kronig relations and varies between 2.9 (x = 1) and 3.5 (x = 0). This ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium
Gallium is a chemical element with the Symbol (chemistry), symbol Ga and atomic number 31. Discovered by France, French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in boron group, group 13 of the periodic table and is similar to the other metals of the group (aluminium, indium, and thallium). Elemental gallium is a soft, silvery metal in Standard conditions for temperature and pressure, standard temperature and pressure. In its liquid state, it becomes silvery white. If too much force is applied, the gallium may fracture conchoidal fracture, conchoidally. Since its discovery in 1875, gallium has widely been used to make alloys with low melting points. It is also used in semiconductors, as a dopant in semiconductor substrates. The melting point of gallium is used as a temperature reference point. Gallium alloys are used in thermometers as a non-toxic and environmentally friendly alternative to mercury, and can withstand higher temperatures than mercury. An even lo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microwave
Microwave is a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter corresponding to frequencies between 300 MHz and 300 GHz respectively. Different sources define different frequency ranges as microwaves; the above broad definition includes both UHF and EHF (millimeter wave) bands. A more common definition in radio-frequency engineering is the range between 1 and 100 GHz (wavelengths between 0.3 m and 3 mm). In all cases, microwaves include the entire SHF band (3 to 30 GHz, or 10 to 1 cm) at minimum. Frequencies in the microwave range are often referred to by their IEEE radar band designations: S, C, X, Ku, K, or Ka band, or by similar NATO or EU designations. The prefix ' in ''microwave'' is not meant to suggest a wavelength in the micrometer range. Rather, it indicates that microwaves are "small" (having shorter wavelengths), compared to the radio waves used prior to microwave ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monolithic Microwave Integrated Circuit
Monolithic microwave integrated circuit, or MMIC (sometimes pronounced "mimic"), is a type of integrated circuit (IC) device that operates at microwave frequencies (300 MHz to 300 GHz). These devices typically perform functions such as microwave mixing, power amplification, low-noise amplification, and high-frequency switching. Inputs and outputs on MMIC devices are frequently matched to a characteristic impedance of 50 ohms. This makes them easier to use, as cascading of MMICs does not then require an external matching network. Additionally, most microwave test equipment is designed to operate in a 50-ohm environment. MMICs are dimensionally small (from around 1 mm² to 10 mm²) and can be mass-produced, which has allowed the proliferation of high-frequency devices such as cellular phones. MMICs were originally fabricated using gallium arsenide (GaAs), a III-V compound semiconductor. It has two fundamental advantages over silicon (Si), the traditional mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MOCVD
Metalorganic vapour-phase epitaxy (MOVPE), also known as organometallic vapour-phase epitaxy (OMVPE) or metalorganic chemical vapour deposition (MOCVD), is a chemical vapour deposition method used to produce single- or polycrystalline thin films. It is a process for growing crystalline layers to create complex semiconductor multilayer structures. In contrast to molecular-beam epitaxy (MBE), the growth of crystals is by chemical reaction and not physical deposition. This takes place not in vacuum, but from the gas phase at moderate pressures (10 to 760 Torr). As such, this technique is preferred for the formation of devices incorporating thermodynamically metastable alloys, and it has become a major process in the manufacture of optoelectronics, such as Light-emitting diodes. It was invented in 1968 at North American Aviation (later Rockwell International) Science Center by Harold M. Manasevit. Basic principles In MOCVD ultrapure precursor gases are injected into a reactor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Beam Epitaxy
Molecular-beam epitaxy (MBE) is an epitaxy method for thin-film deposition of single crystals. MBE is widely used in the manufacture of semiconductor devices, including transistors, and it is considered one of the fundamental tools for the development of nanotechnologies. MBE is used to fabricate diodes and MOSFETs (MOS field-effect transistors) at microwave frequencies, and to manufacture the lasers used to read optical discs (such as CDs and DVDs). History Original ideas of MBE process were first established by Günther. Films he deposited were not epitaxial, but were deposited on glass substrates. With the development of vacuum technology, MBE process was demonstrated by Davey and Pankey who succeeded in growing GaAs epitaxial films on single crystal GaAs substrates using Günther's method. Major subsequent development of MBE films was enabled by J.R. Arthur's investigations of kinetic behavior of growth mechanisms and Alfred Y. Cho's in situ observation of MBE process ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsine
Arsine (IUPAC name: arsane) is an inorganic compound with the formula As H3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications in the semiconductor industry and for the synthesis of organoarsenic compounds. The term ''arsine'' is commonly used to describe a class of organoarsenic compounds of the formula AsH3−xRx, where R = aryl or alkyl. For example, As(C6H5)3, called triphenylarsine, is referred to as "an arsine". General properties At its standard state, arsine is a colorless, denser-than-air gas that is slightly soluble in water (20% at 20 °C) and in many organic solvents as well. Whereas arsine itself is odorless, owing to its oxidation by air, it is possible to smell a slight garlic or fish-like scent when the compound is present above 0.5 ppm. This compound is kinetically stable: at room temperature it decomposes only slowly. At temperatures of ca. 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |