|
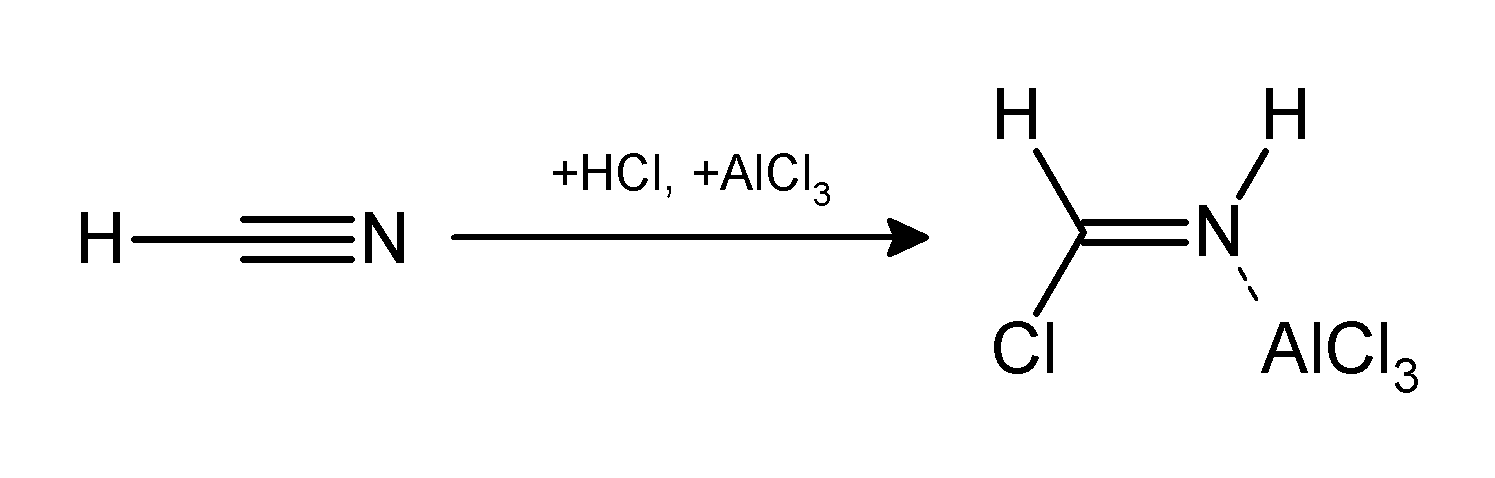
Gatterman-Koch Reaction
The Gattermann reaction (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) in the presence of a Lewis acid catalyst such as aluminium chloride (AlCl3). It is named for the German chemist Ludwig Gattermann and is similar to the Friedel–Crafts reaction. Modifications have shown that it is possible to use sodium cyanide or cyanogen bromide in place of hydrogen cyanide. The reaction can be simplified by replacing the HCN/AlCl3 combination with zinc cyanide. Although it is also highly toxic, Zn(CN)2 is a solid, making it safer to work with than gaseous HCN. The Zn(CN)2 reacts with the HCl to form the key HCN reactant and Zn(Cl)2 that serves as the Lewis-acid catalyst ''in-situ''. An example of the Zn(CN)2 method is the synthesis of mesitaldehyde from mesitylene. Gattermann–Koch reaction The Gattermann– ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ludwig Gattermann
Ludwig Gattermann (20 April 1860 – 20 June 1920) was a German chemist who contributed significantly to both organic and inorganic chemistry. Early life Ludwig Gatterman was born on 20 April 1860 in Goslar, an old mining town north of the Harz mountains. Two of his three siblings died at a young age. During his time in the Realschule he started experimenting. In 1880, he wanted to study at the University of Leipzig, but he had to complete his compulsory military service before he could start. He started his studies in 1881. After one year with Robert Bunsen at the University of Leipzig, he visited Liebermann for one semester at the University of Berlin to improve his skills in organic chemistry. Gattermann chose the University of Göttingen, which was close to Goslar for his further studies. He started his thesis under the supervision of Hans Hübner, who died in 1884, and finished his Ph.D. in 1885. As successor of Hans Hübner, Victor Meyer came to Göttingen and some re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemische Berichte
''Chemische Berichte'' (usually abbreviated as ''Ber.'' or ''Chem. Ber.'') was a German-language scientific journal of all disciplines of chemistry founded in 1868. It was one of the oldest scientific journals in chemistry, until it merged with '' Recueil des Travaux Chimiques des Pays-Bas'' to form ''Chemische Berichte/Recueil'' in 1997. ''Chemische Berichte/Recueil'' was then merged with other European journals in 1998 to form ''European Journal of Inorganic Chemistry''. History Founded in 1868 as ''Berichte der Deutschen Chemischen Gesellschaft'' (, CODEN BDCGAS), it operated under this title until 1928 (Vol. 61). The journal was then split into: * ''Berichte der Deutschen Chemischen Gesellschaft, A: Vereins-Nachrichten'' (, CODEN BDCAAS), and * ''Berichte der Deutschen Chemischen Gesellschaft, B: Abhandlungen'' (, CODEN BDCBAD). Vol. 78 and 79 (1945–1946) were omitted and not published due to World War II. The journal was renamed ''Chemische Berichte'' (, CODEN CHBEAM) in 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formylation Reactions
Formylation refers to any chemical processes in which a compound is functionalized with a formyl group (-CH=O). In organic chemistry, the term is most commonly used with regards to aromatic compounds (for example the conversion of benzene to benzaldehyde in the Gattermann–Koch reaction). In biochemistry the reaction is catalysed by enzymes such as formyltransferases. Formylation generally involves the use of formylation agents, reagents that give rise to the CHO group. Among the many formylation reagents, particularly important are formic acid and carbon monoxide. A formylation reaction in organic chemistry refers to organic reactions in which an organic compound is functionalized with a formyl group (-CH=O). The reaction is a route to aldehydes (''C''-CH=O), formamides (''N''-CH=O), and formate#Formate esters, formate esters (''O''-CH=O). Formylation agents A reagent that delivers the formyl group is called a formylating agent. * Formic acid * Dimethylformamide and phosphorus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Name Reactions
A name reaction (or named reaction) is a chemical reaction named after its discoverer(s) or developer(s). Among the tens of thousands of organic reactions that are known, hundreds of such reactions are typically identified by the eponym. Well-known examples include the Grignard reaction, the Sabatier reaction, the Wittig reaction, the Claisen condensation, the Friedel–Crafts acylation, and the Diels–Alder reaction. Books have been published devoted exclusively to name reactions;Alfred Hassner, C. Stumer. ''Organic syntheses based on name reactions''. Elsevier, 2002. Li, Jie Jack. ''Name Reactions: A Collection of Detailed Reaction Mechanisms''. Springer, 2003. the ''Merck Index ''The Merck Index'' is an encyclopedia of chemical substance, chemicals, pharmaceutical drug, drugs and biomolecule, biologicals with over 10,000 monographs on single substances or groups of related chemical compound, compounds published online ...'', a chemical encyclopedia, also includes an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substitution Reactions
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions are of prime importance in organic chemistry. Substitution reactions in organic chemistry are classified either as electrophile, electrophilic or nucleophile, nucleophilic depending upon the reagent involved, whether a reactive intermediate involved in the reaction is a carbocation, a carbanion or a radical (chemistry), free radical, and whether the substrate (chemistry), substrate is aliphatic or aromatic. Detailed understanding of a reaction type helps to predict the product outcome in a reaction. It also is helpful for optimizing a reaction with regard to variables such as temperature and choice of solvent. A good example of a substitution reaction is halogenation. When chlorine gas (Cl2) is irradiated, some of the molecules are split int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stephen Aldehyde Synthesis
Stephen aldehyde synthesis, a named reaction in chemistry, was invented by Henry Stephen (chemist), Henry Stephen (Order of the British Empire, OBE/Member of the Order of the British Empire, MBE). This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R-CN) using tin(II) chloride (SnCl2), hydrochloric acid (HCl) and quenching the resulting iminium salt ([R-CH=NH2]+Cl−) with Water (molecule), water (H2O). During the synthesis, ammonium chloride is also produced. It is a type of nucleophilic addition reaction. Mechanism The following scheme shows the reaction mechanism: By addition of hydrogen chloride the used nitrile (1) reacts to its corresponding salt (2). It is believed that this salt is reduced by a single electron transfer by the tin(II) chloride (3a and 3b). The resulting salt (4) precipitates after some time as aldimine tin chloride (5). Hydrolysis of 5 produces a hemiaminal (6) from which an aldehyde (7) is formed. Substitutes that increase the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Houben–Hoesch Reaction
The Hoesch reaction or Houben–Hoesch reaction is an organic reaction in which a nitrile reacts with an arene compound to form an aryl ketone. The reaction is a type of Friedel–Crafts acylation with hydrogen chloride and a Lewis acid catalyst. The synthesis of 2,4,6-Trihydroxyacetophenone (THAP) from phloroglucinol is representative: If two-equivalents are added, 2,4-Diacetylphloroglucinol is the product. : An imine can be isolated as an intermediate reaction product. The attacking electrophile is possibly a species of the type R-C+=NHCl−. The arene must be electron-rich i.e. phenol or aniline type. A related reaction is the Gattermann reaction in which hydrocyanic acid not a nitrile is used. The reaction is named after Kurt Hoesch and Josef Houben''Über die Kern-Kondensation von Phenolen und Phenol-äthern mit Nitrilen zu Phenol- und Phenol-äther-Ketimiden und -Ketonen (I.)'' Berichte der deutschen chemischen Gesellschaft (A and B Series) Volume 59, Issue 11, Date: 8. De ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel(II) Chloride
Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. The nickel chlorides are deliquescent, absorbing moisture from the air to form a solution. Nickel salts have been shown to be carcinogenic to the lungs and nasal passages in cases of long-term inhalation exposure. Production and syntheses Large scale production and uses of nickel chloride are associated with the purification of nickel from its ores. It is generated upon extraction nickel matte and residues obtained from roasting refining nickel-containing ores using hydrochloric acid. Electrolysis of nickel chloride solutions are used in the production of nickel metal. Other significant routes to nickel chloride arise from processing of ore concentrates such as various reactions involving copper chlorides: : : Labo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(I) Chloride
Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear green due to the presence of copper(II) chloride (CuCl2). History Copper(I) chloride was first prepared by Robert Boyle and designated rosin of copper in the mid-seventeenth century from mercury(II) chloride ("Venetian sublimate") and copper metal: :HgCl2 + 2 Cu → 2 CuCl + Hg In 1799, Joseph Proust first differentiated two different chlorides of copper. He prepared CuCl (which he called white muriate of copper) by heating CuCl2 at red heat in the absence of air, causing it to lose half of its combined chlorine followed by removing residual CuCl2 by washing with water. An acidic solution of CuCl was formerly used to analyze carbon monoxide content in gases, for example in Hempel's gas apparatus where the CuCl absorbs the carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R′ represent the organyl groups. Ethers can again be classified into two varieties: if the organyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin. Structure and bonding Ethers feature bent linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of ox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. It is acutely toxic and is considered a health hazard. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 million tonnes a year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds, and is a liquid when manufactured. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, explosives such as picric acid, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceuti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |