|
Excess Chemical Potential
In thermodynamics, the excess chemical potential is defined as the difference between the chemical potential of a given species and that of an ideal gas under the same conditions (in particular, at the same pressure, temperature, and composition). The chemical potential of a particle species i is therefore given by an ideal part and an excess part. :\mu_i=\mu_i^\text+\mu_i^\text Chemical potential of a pure fluid can be estimated by the Widom insertion method. Derivation and Measurement For a system of diameter and volume , at constant temperature , the classical canonical partition function :Q(N,V,T)=\frac\int_^\ldots\int_^ds^\exp \beta U(s^;L)/math> with a scaled coordinate, the free energy is given by: :F(N,V,T)= -k_T\ln Q=-k_T\ln\left(\frac\right)-k_T \ln= :=F_(N,V,T)+F_(N,V,T) Combining the above equation with the definition of chemical potential, :\mu_= \left(\frac\right)_=\left(\frac\right)_, we get the chemical potential of a sufficiently large system from (and th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to formula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Free Energy
The thermodynamic free energy is a concept useful in the thermodynamics of chemical or thermal processes in engineering and science. The change in the free energy is the maximum amount of work that a thermodynamic system can perform in a process at constant temperature, and its sign indicates whether the process is thermodynamically favorable or forbidden. Since free energy usually contains potential energy, it is not absolute but depends on the choice of a zero point. Therefore, only relative free energy values, or changes in free energy, are physically meaningful. The free energy is a thermodynamic state function, like the internal energy, enthalpy, and entropy. The free energy is the portion of any first-law energy that is available to perform thermodynamic work at constant temperature, ''i.e.'', work mediated by thermal energy. Free energy is subject to irreversible loss in the course of such work. Since first-law energy is always conserved, it is evident that free energ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to formula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potentials
Potential generally refers to a currently unrealized ability, in a wide variety of fields from physics to the social sciences. Mathematics and physics * Scalar potential, a scalar field whose gradient is a given vector field * Vector potential, a vector field whose curl is a given vector field * Potential function (other) * Potential variable (Boolean differential calculus) * Potential energy, the energy possessed by an object because of its position relative to other objects, stresses within itself, its electric charge, or other factors * Magnetic vector potential * Magnetic scalar potential (ψ) * Electric potential, the amount of work needed to move a unit positive charge from a reference point to a specific point inside the field without producing any acceleration * Electromagnetic four-potential, a relativistic vector function from which the electromagnetic field can be derived * Coulomb potential * Van der Waals force, distance-dependent interactions between a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copyright Problems
A copyright is a type of intellectual property that gives its owner the exclusive right to copy, distribute, adapt, display, and perform a creative work, usually for a limited time. The creative work may be in a literary, artistic, educational, or musical form. Copyright is intended to protect the original expression of an idea in the form of a creative work, but not the idea itself. A copyright is subject to limitations based on public interest considerations, such as the fair use doctrine in the United States. Some jurisdictions require "fixing" copyrighted works in a tangible form. It is often shared among multiple authors, each of whom holds a set of rights to use or license the work, and who are commonly referred to as rights holders. These rights frequently include reproduction, control over derivative works, distribution, public performance, and moral rights such as attribution. Copyrights can be granted by public law and are in that case considered "territorial rig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apparent Molar Property
In thermodynamics, an apparent molar property of a solution component in a mixture or solution is a quantity defined with the purpose of isolating the contribution of each component to the non-ideality of the mixture. It shows the change in the corresponding solution property (for example, volume) per mole of that component added, when all of that component is added to the solution. It is described as ''apparent'' because it appears to represent the molar property of that component ''in solution'', provided that the properties of the other solution components are assumed to remain constant during the addition. However this assumption is often not justified, since the values of apparent molar properties of a component may be quite different from its molar properties in the pure state. For instance, the volume of a solution containing two components identified as solvent and solute is given by : V=V_0 + ^\phi_1 \ =\tilde_ n_ + ^\phi\tilde_1 n_1 \, where is the volume of the pure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microcanonical Ensemble
In statistical mechanics, the microcanonical ensemble is a statistical ensemble that represents the possible states of a mechanical system whose total energy is exactly specified. The system is assumed to be isolated in the sense that it cannot exchange energy or particles with its environment, so that (by conservation of energy) the energy of the system does not change with time. The primary macroscopic variables of the microcanonical ensemble are the total number of particles in the system (symbol: ), the system's volume (symbol: ), as well as the total energy in the system (symbol: ). Each of these is assumed to be constant in the ensemble. For this reason, the microcanonical ensemble is sometimes called the ensemble. In simple terms, the microcanonical ensemble is defined by assigning an equal probability to every microstate whose energy falls within a range centered at . All other microstates are given a probability of zero. Since the probabilities must add up to 1, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Statistical Ensemble (mathematical Physics)
In physics, specifically statistical mechanics, an ensemble (also statistical ensemble) is an idealization consisting of a large number of virtual copies (sometimes infinitely many) of a system, considered all at once, each of which represents a possible state that the real system might be in. In other words, a statistical ensemble is a set of systems of particles used in statistical mechanics to describe a single system. The concept of an ensemble was introduced by J. Willard Gibbs in 1902. A thermodynamic ensemble is a specific variety of statistical ensemble that, among other properties, is in statistical equilibrium (defined below), and is used to derive the properties of thermodynamic systems from the laws of classical or quantum mechanics. Physical considerations The ensemble formalises the notion that an experimenter repeating an experiment again and again under the same macroscopic conditions, but unable to control the microscopic details, may expect to observe a ran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monte Carlo Method
Monte Carlo methods, or Monte Carlo experiments, are a broad class of computational algorithms that rely on repeated random sampling to obtain numerical results. The underlying concept is to use randomness to solve problems that might be deterministic in principle. They are often used in physical and mathematical problems and are most useful when it is difficult or impossible to use other approaches. Monte Carlo methods are mainly used in three problem classes: optimization, numerical integration, and generating draws from a probability distribution. In physics-related problems, Monte Carlo methods are useful for simulating systems with many coupled degrees of freedom, such as fluids, disordered materials, strongly coupled solids, and cellular structures (see cellular Potts model, interacting particle systems, McKean–Vlasov processes, kinetic models of gases). Other examples include modeling phenomena with significant uncertainty in inputs such as the calculation of ris ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partition Function (statistical Mechanics)
In physics, a partition function describes the statistical properties of a system in thermodynamic equilibrium. Partition functions are functions of the thermodynamic state variables, such as the temperature and volume. Most of the aggregate thermodynamic variables of the system, such as the total energy, free energy, entropy, and pressure, can be expressed in terms of the partition function or its derivatives. The partition function is dimensionless. Each partition function is constructed to represent a particular statistical ensemble (which, in turn, corresponds to a particular free energy). The most common statistical ensembles have named partition functions. The canonical partition function applies to a canonical ensemble, in which the system is allowed to exchange heat with the environment at fixed temperature, volume, and number of particles. The grand canonical partition function applies to a grand canonical ensemble, in which the system can exchange both heat and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
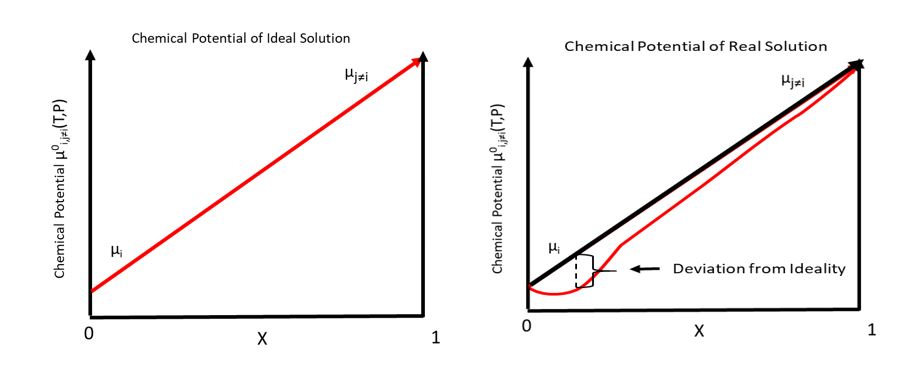
Chemical Potential
In thermodynamics, the chemical potential of a species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potential of a species in a mixture is defined as the rate of change of free energy of a thermodynamic system with respect to the change in the number of atoms or molecules of the species that are added to the system. Thus, it is the partial derivative of the free energy with respect to the amount of the species, all other species' concentrations in the mixture remaining constant. When both temperature and pressure are held constant, and the number of particles is expressed in moles, the chemical potential is the partial molar Gibbs free energy. At chemical equilibrium or in phase equilibrium, the total sum of the product of chemical potentials and stoichiometric coefficients is zero, as the free energy is at a minimum. In a system in diffusion equilibrium, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Widom Insertion Method
The Widom insertion method is a statistical thermodynamic approach to the calculation of material and mixture properties. It is named for Benjamin Widom, who derived it in 1963.Widom, B, "Some Topics in the Theory of Fluids", ''J. Chem. Phys.'', 1963, 39(11), 2808-2812. In general, there are two theoretical approaches to determining the statistical mechanical properties of materials. The first is the direct calculation of the overall partition function of the system, which directly yields the system free energy. The second approach, known as the Widom insertion method, instead derives from calculations centering on one molecule. The Widom insertion method directly yields the chemical potential of one component rather than the system free energy. This approach is most widely applied in molecular computer simulations but has also been applied in the development of analytical statistical mechanical models. The Widom insertion method can be understood as an application of the Jar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)