|
E-4 Process
:''See also Ektachrome for full details of Kodak E-series processes.'' The E-4 process is a now outdated process for developing color reversal (transparency) photographic film, that was introduced in 1966. The process is infamous for two reasons: First, its use of the highly toxic reversal agent tertiary butyl-amine borane (TBAB) (not to be confused with tetra-n-butylammonium bromide, which also has TBAB as abbr.) – as of all boron hydrides. Early releases of the consumer-sized version of the chemistry provided the TBAB in the form of a tablet. This was later changed to loose powder (likely as a countermeasure against inadvertent ingestion of the substance). The use of the reversal agent permits processing of the film without the manual reexposure that its predecessor E-3 required. Kodak's official E-6 process, which replaced Process E-4 for almost all applications, avoids the necessity of TBAB by adding a separate reversal bath containing the tin salt stannous chloride Tin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
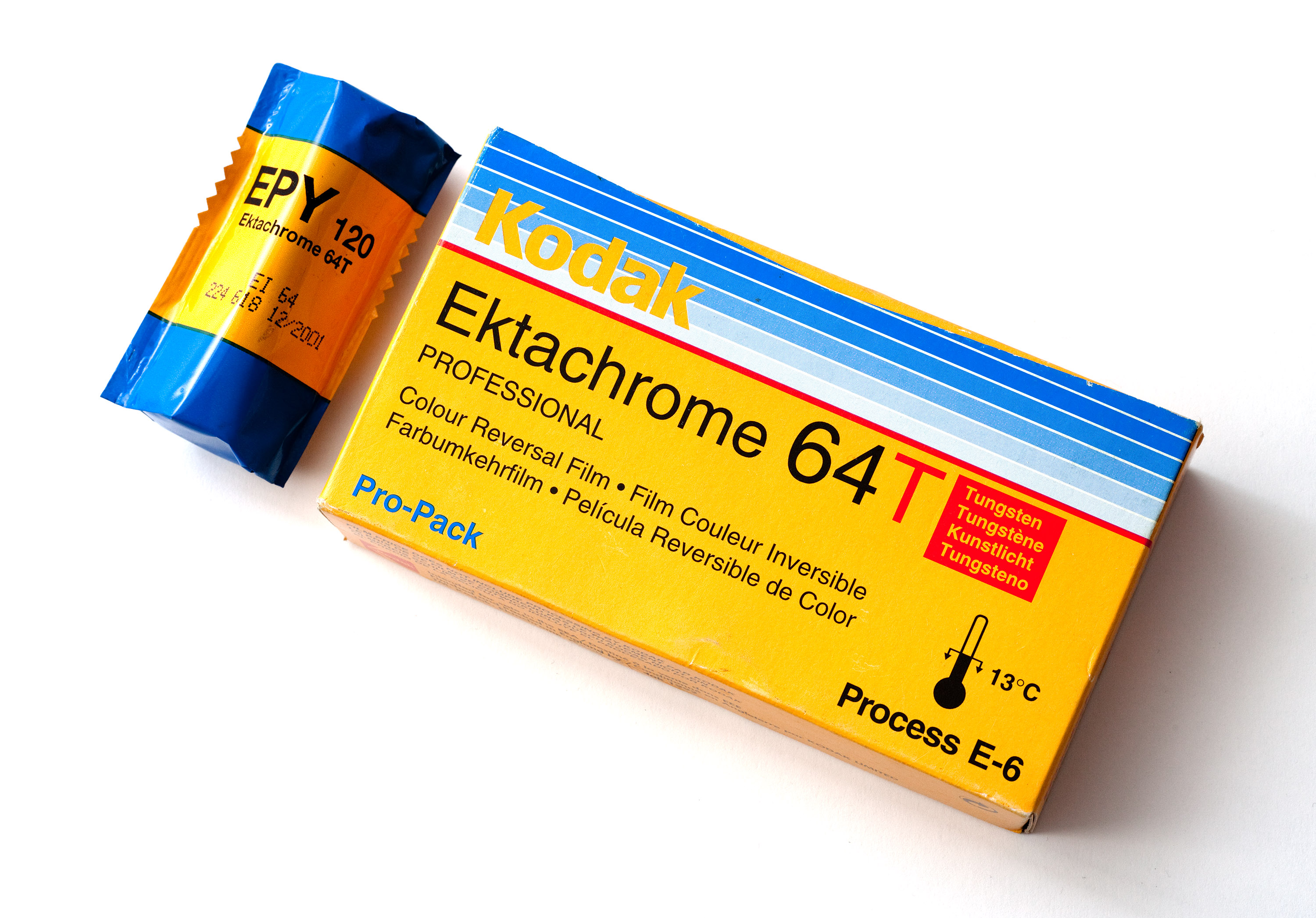
Ektachrome
Ektachrome is a brand name owned by Kodak for a range of transparency, still, and motion picture films previously available in many formats, including 35 mm and sheet sizes to 11 × 14 inch size. Ektachrome has a distinctive look that became familiar to many readers of ''National Geographic'', which used it extensively for color photographs for decades in settings where Kodachrome was too slow. In terms of reciprocity characteristics, Ektachrome is stable at shutter speeds between ten seconds and 1/10,000 of a second. Ektachrome, initially developed in the early 1940s, allowed professionals and amateurs alike to process their own films. It also made color reversal film more practical in larger formats, and the Kodachrome Professional film in sheet sizes was later discontinued. High Speed Ektachrome, announced in 1959 provided an ASA 160 color film, which was much faster than Kodachrome. In 1968, Kodak started offering push processing of this film, allowing it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transparency (photography)
In photography, reversal film or slide film is a type of photographic film that produces a positive image on a transparent base. Instead of negatives and prints, reversal film is processed to produce transparencies or diapositives (abbreviated as "diafilm" or "dia" in some languages like German or Hungarian). Reversal film is produced in various sizes, from 35 mm to roll film to 8×10 inch sheet film. A slide is a specially mounted individual transparency intended for projection onto a screen using a slide projector. This allows the photograph to be viewed by a large audience at once. The most common form is the 35 mm slide, with the image framed in a 2×2 inch cardboard or plastic mount. Some specialized labs produce photographic slides from digital camera images in formats such as JPEG, from computer-generated presentation graphics, and from a wide variety of physical source material such as fingerprints, microscopic sections, paper documents, astronomical images ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tertiary Butyl-amine Borane
Borane ''tert''-butylamine is an amine borane complex derived from ''tert''-butylamine and borane. It is a colorless solid. The compound is prepared by the reaction of tert-butylammonium chloride and sodium borohydride:Girolami, G. S.; Rauchfuss, T. B. and Angelici, R. J., Synthesis and Technique in Inorganic Chemistry, University Science Books: Mill Valley, CA, 1999. :''t''-BuNH3Cl + NaBH4 → ''t''-BuNH2BH3 + H2 + NaCl In organic synthesis, borane ''tert-''butylamine can be used for selective reduction of certain functional groups including aldehydes, ketones, oximes, and imines. In photographic processing Photographic processing or photographic development is the chemical means by which photographic film or paper is treated after photographic exposure to produce a negative or positive image. Photographic processing transforms the latent image in ..., it is used in the E-4 process, as "chemical enlighting" step in the processing of the film. See also * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetra-n-butylammonium Bromide
Tetrabutylammonium bromide (TBAB) is a quaternary ammonium salt with a bromide commonly used as a phase transfer catalyst. It is used to prepare many other tetrabutylammonium salts by salt metathesis reactions. The anhydrous form is a white solid. In addition to being cheap, tetrabutylammonium bromide is also environmentally friendly, has a greater degree of selectivity, is operationally simple, non-corrosive, and can be recycled easily as well. Preparation and reactions Tetrabutylammonium bromide can be prepared by the alkylation of tributylamine with 1-bromobutane. Tetrabutylammonium bromide is used to prepare other salts of the tetrabutylammonium cation by salt metathesis reactions., , ;. It serves as a source of bromide ions for substitution reactions. It is one of a commonly-used phase transfer catalyst. As its melting point is just over 100 °C and decreases in the presence of other reagents, it can be considered an ionic liquid. Role in semi-clathrate formation TBAB ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E-3 Process
:''See also Ektachrome for full details of Kodak E-series processes.'' The E-2 process and E-3 process are outdated processes for developing Ektachrome reversal photographic film. The two processes are very similar, and differ depending on the film. Kodak sold kits that could process either kind of film. Films are processed at 75°F (23.9°C) with a tolerance of only 0.5°F. The steps are: *First developer. This is a conventional black-and-white developer, and develops as a negative. *Stop bath *Hardener After this, the film is removed from the tank and thoroughly exposed with a bright light (Photoflood Photoflood lamps are a type of incandescent light bulb designed for use as a continuous light source for photographic Photography is the art, application, and practice of creating durable images by recording light, either electronically by mea ...). Replace in tank, though the lid was no longer required. *Colour developer. This develops the now exposed silver bromid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E-6 Process
The E-6 process (often abbreviated to E-6) is a chromogenic photographic process for developing Ektachrome, Fujichrome and other color reversal (also called slide or transparency) photographic film. Unlike some color reversal processes (such as Kodachrome K-14) that produce positive transparencies, E-6 processing can be performed by individual users with the same equipment that is used for processing black and white negative film or C-41 color negative film. The process is highly sensitive to temperature variations: A heated water bath is mandatory to stabilize the temperature at 100.0 °F (37.8 °C) for the first developer and first wash to maintain process tolerances. History The E-6 process superseded Kodak's E-3 and E-4 processes. The E-3 process required fogging with light to accomplish image reversal and produced transparencies that faded quickly. The E-4 process used polluting chemicals, such as the highly toxic reversal agent borane tert-butylamine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stannous Chloride
Tin(II) chloride, also known as stannous chloride, is a white crystalline solid with the formula . It forms a stable dihydrate, but aqueous solutions tend to undergo hydrolysis, particularly if hot. SnCl2 is widely used as a reducing agent (in acid solution), and in electrolytic baths for tin-plating. Tin(II) chloride should not be confused with the other chloride of tin; tin(IV) chloride or stannic chloride (SnCl4). Chemical structure SnCl2 has a lone pair of electrons, such that the molecule in the gas phase is bent. In the solid state, crystalline SnCl2 forms chains linked via chloride bridges as shown. The dihydrate is also three-coordinate, with one water coordinated on to the tin, and a second water coordinated to the first. The main part of the molecule stacks into double layers in the crystal lattice, with the "second" water sandwiched between the layers. Chemical properties Tin(II) chloride can dissolve in less than its own mass of water without apparent decompositi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


-chloride-xtal-1996-3D-balls-front.png)