|
DLVO Theory
The DLVO theory (named after Boris Derjaguin and Lev Landau, Evert Verwey and Theodoor Overbeek) explains the aggregation of aqueous dispersions quantitatively and describes the force between charged surfaces interacting through a liquid medium. It combines the effects of the van der Waals attraction and the electrostatic repulsion due to the so-called double layer of counterions. The electrostatic part of the DLVO interaction is computed in the mean field approximation in the limit of low surface potentials - that is when the potential energy of an elementary charge on the surface is much smaller than the thermal energy scale, k_ T. For two spheres of radius a each having a charge Z (expressed in units of the elementary charge) separated by a center-to-center distance r in a fluid of dielectric constant \epsilon_r containing a concentration n of monovalent ions, the electrostatic potential takes the form of a screened-Coulomb or Yukawa potential, : \beta U(r) = Z^2 \lambda_ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boris Derjaguin
Boris Vladimirovich Derjaguin (or Deryagin; russian: Бори́с Влади́мирович Деря́гин) (9 August 1902 in Moscow – 16 May 1994) was a Soviet and Russian chemist. As a member of the Russian Academy of Sciences, he laid the foundation of the modern science of colloids and surfaces. An epoch in the development of the physical chemistry of colloids and surfaces is associated with his name. Derjaguin became famous in scientific circles for his work on the stability of colloids and thin films of liquids which is now known as the DLVO theory, after the initials of its authors: Derjaguin, Landau, Verwey, and Overbeek. It is universally included in text books on colloid chemistry and is still widely applied in modern studies of interparticle forces in colloids. In particular, the Derjaguin approximation is widely used in order to approximate the interaction between curved surfaces from a knowledge of the interaction for planar ones. Derjaguin was also briefly invol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy Barrier
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as the magnitude of the potential barrier (sometimes called the energy barrier) separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term "activation energy" was introduced in 1889 by the Swedish scientist Svante Arrhenius. Other uses Although less commonly used, activation energy also applies to nuclear reactions and various other physical phenomena. Te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluid Dynamic
In physics and engineering, fluid dynamics is a subdiscipline of fluid mechanics that describes the flow of fluids— liquids and gases. It has several subdisciplines, including ''aerodynamics'' (the study of air and other gases in motion) and hydrodynamics (the study of liquids in motion). Fluid dynamics has a wide range of applications, including calculating forces and moments on aircraft, determining the mass flow rate of petroleum through pipelines, predicting weather patterns, understanding nebulae in interstellar space and modelling fission weapon detonation. Fluid dynamics offers a systematic structure—which underlies these practical disciplines—that embraces empirical and semi-empirical laws derived from flow measurement and used to solve practical problems. The solution to a fluid dynamics problem typically involves the calculation of various properties of the fluid, such as flow velocity, pressure, density, and temperature, as functions of space and time. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shear Flow
The term shear flow is used in solid mechanics as well as in fluid dynamics. The expression ''shear flow'' is used to indicate: * a shear stress over a distance in a thin-walled structure (in solid mechanics);Higdon, Ohlsen, Stiles and Weese (1960), ''Mechanics of Materials'', article 4-9 (2nd edition), John Wiley & Sons, Inc., New York. Library of Congress CCN 66-25222 * the flow ''induced'' by a force (in a fluid). In solid mechanics For thin-walled profiles, such as that through a beam or semi-monocoque structure, the shear stress distribution through the thickness can be neglected. Furthermore, there is no shear stress in the direction normal to the wall, only parallel. In these instances, it can be useful to express internal shear stress as shear flow, which is found as the shear stress multiplied by the thickness of the section. An equivalent definition for shear flow is the shear force ''V'' per unit length of the perimeter around a thin-walled section. Shear flow has the d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann Constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constant, and in Planck's law of black-body radiation and Boltzmann's entropy formula, and is used in calculating thermal noise in resistors. The Boltzmann constant has dimensions of energy divided by temperature, the same as entropy. It is named after the Austrian scientist Ludwig Boltzmann. As part of the 2019 redefinition of SI base units, the Boltzmann constant is one of the seven " defining constants" that have been given exact definitions. They are used in various combinations to define the seven SI base units. The Boltzmann constant is defined to be exactly . Roles of the Boltzmann constant Macroscopically, the ideal gas law states that, for an ideal gas, the product of pressure and volume is proportional to the product of amount ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Relative Static Permittivity
The relative permittivity (in older texts, dielectric constant) is the permittivity of a material expressed as a ratio with the electric permittivity of a vacuum. A dielectric is an insulating material, and the dielectric constant of an insulator measures the ability of the insulator to store electric energy in an electrical field. Permittivity is a material's property that affects the Coulomb force between two point charges in the material. Relative permittivity is the factor by which the electric field between the charges is decreased relative to vacuum. Likewise, relative permittivity is the ratio of the capacitance of a capacitor using that material as a dielectric, compared with a similar capacitor that has vacuum as its dielectric. Relative permittivity is also commonly known as the dielectric constant, a term still used but deprecated by standards organizations in engineering as well as in chemistry. Definition Relative permittivity is typically denoted as (sometimes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum Permittivity
Vacuum permittivity, commonly denoted (pronounced "epsilon nought" or "epsilon zero"), is the value of the absolute dielectric permittivity of classical vacuum. It may also be referred to as the permittivity of free space, the electric constant, or the distributed capacitance of the vacuum. It is an ideal (baseline) physical constant. Its CODATA value is: : (farads per meter), with a relative uncertainty of It is a measure of how dense of an electric field is "permitted" to form in response to electric charges, and relates the units for electric charge to mechanical quantities such as length and force. For example, the force between two separated electric charges with spherical symmetry (in the vacuum of classical electromagnetism) is given by Coulomb's law: :F_\text = \frac \frac Here, ''q''1 and ''q''2 are the charges, ''r'' is the distance between their centres, and the value of the constant fraction 1/4 \pi \varepsilon_0 (known as the Coulomb constant, ''k''e) is ap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
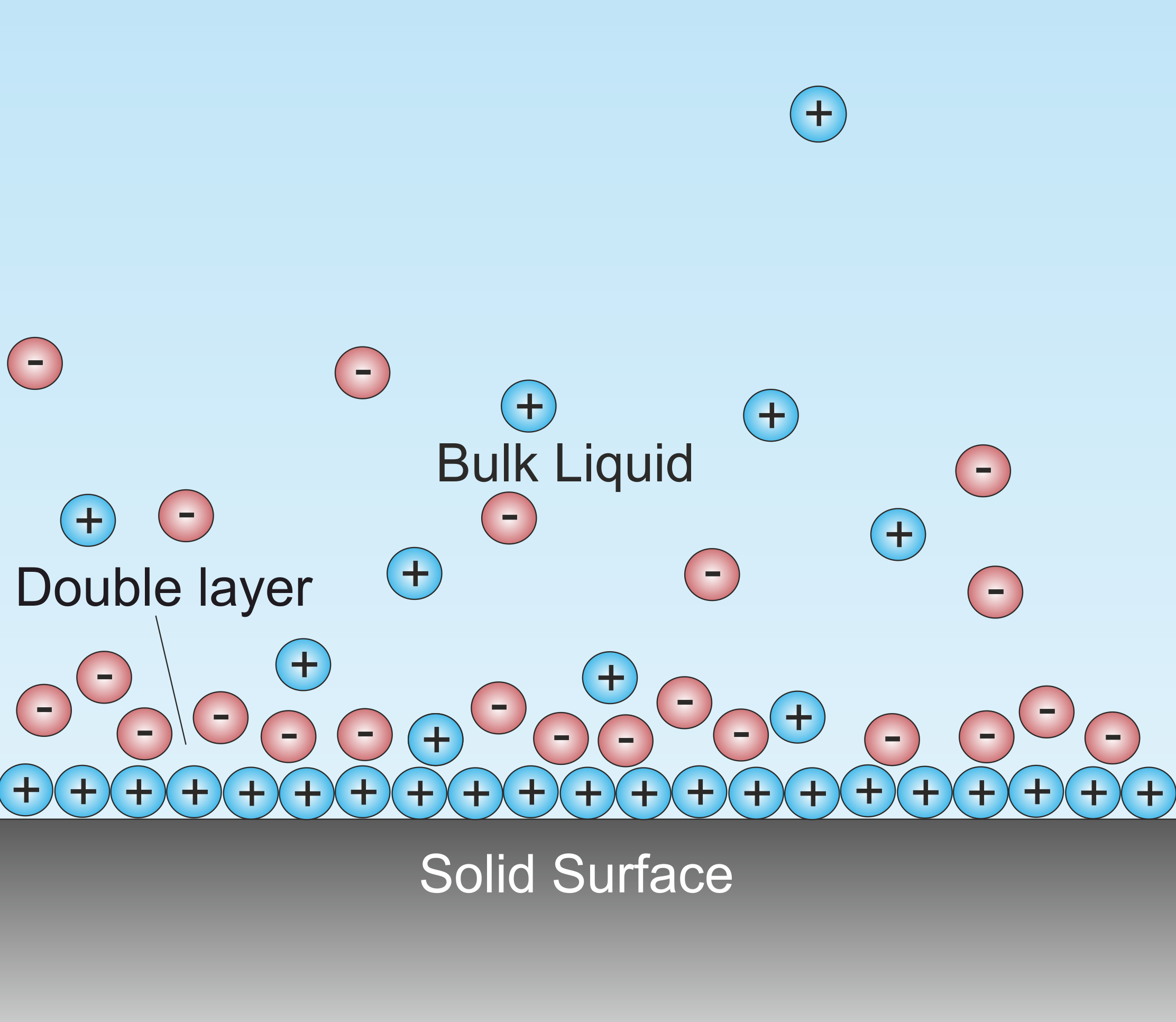
Debye Screening Length
A double layer (DL, also called an electrical double layer, EDL) is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge (either positive or negative), consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer". Interfacial DLs are most apparent in systems with a large surface area to volume ratio, such as a colloid or porous bodies with particles or pores (respectively) on th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibbs Free Energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and pressure. It also provides a necessary condition for processes such as chemical reactions that may occur under these conditions. The Gibbs free energy change , measured in joules in SI) is the ''maximum'' amount of non-expansion work that can be extracted from a closed system (one that can exchange heat and work with its surroundings, but not matter) at fixed temperature and pressure. This maximum can be attained only in a completely reversible process. When a system transforms reversibly from an initial state to a final state under these conditions, the decrease in Gibbs free energy equals the work done by the system to its surroundings, minus the work of the pressure forces. The Gibbs energy is the thermodynamic potential that is m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyelectrolyte
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are thus similar to both electrolytes (salts) and polymers (high molecular weight compounds) and are sometimes called polysalts. Like salts, their solutions are electrically conductive. Like polymers, their solutions are often viscous. Charged molecular chains, commonly present in soft matter systems, play a fundamental role in determining structure, stability and the interactions of various molecular assemblies. Theoretical approaches to describing their statistical properties differ profoundly from those of their electrically neutral counterparts, while technological and industrial fields exploit their unique properties. Many biological molecules are polyelectrolytes. For instance, polypeptides, glycosaminoglycans, and DNA are polyelectroly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Derjaguin Approximation
The Derjaguin approximation (or sometimes also referred to as the proximity approximation), named after the Russian scientist Boris Derjaguin, expresses the force profile acting between finite size bodies in terms of the force profile between two planar semi-infinite walls. This approximation is widely used to estimate forces between colloidal particles, as forces between two planar bodies are often much easier to calculate. The Derjaguin approximation expresses the force ''F''(''h'') between two bodies as a function of the surface separation as : F(h) = 2 \pi R_ W(h), where ''W''(''h'') is the interaction energy per unit area between the two planar walls and ''R''eff the effective radius. When the two bodies are two spheres of radii ''R''1 and ''R''2, respectively, the effective radius is given by : R_^ = R_1^+R_2^. Experimental force profiles between macroscopic bodies as measured with the surface forces apparatus (SFA) or colloidal probe technique are often reported as the r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hamaker Constant
The Hamaker constant ''A'' can be defined for a van der Waals (vdW) body–body interaction: :A=\pi^2C\rho_1\rho_2, where \rho_1 and \rho_2 are the number densities of the two interacting kinds of particles, and ''C'' is the London coefficient in the particle–particle pair interaction. It is named after H. C. Hamaker. The magnitude of this constant reflects the strength of the vdW-force between two particles, or between a particle and a substrate. The Hamaker constant provides the means to determine the interaction parameter ''C'' from the vdW-pair potential, w(r) = -C/r^6. Hamaker's method and the associated Hamaker constant ignores the influence of an intervening medium between the two particles of interaction. In 1956 Lifshitz developed a description of the vdW energy but with consideration of the dielectric properties of this intervening medium (often a continuous phase). The Van der Waals forces are effective only up to several hundred angstroms. When the interactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |