|
Deposition (aerosol Physics)
In the physics of aerosols, deposition is the process by which aerosol particles collect or deposit themselves on solid surfaces, decreasing the concentration of the particles in the air. It can be divided into two sub-processes: ''dry'' and ''wet'' deposition. The rate of deposition, or the deposition velocity, is slowest for particles of an intermediate size. Mechanisms for deposition are most effective for either very small or very large particles. Very large particles will settle out quickly through sedimentation (settling) or impaction processes, while Brownian diffusion has the greatest influence on small particles. This is because very small particles coagulate in few hours until they achieve a diameter of 0.5 micrometres. At this size they no longer coagulate. This has a great influence in the amount of PM-2.5 present in the air. ''Deposition velocity'' is defined from , where is flux density, is deposition velocity and is concentration. In gravitational deposit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physics
Physics is the scientific study of matter, its Elementary particle, fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which relates to the order of nature, or, in other words, to the regular succession of events." It is one of the most fundamental scientific disciplines. "Physics is one of the most fundamental of the sciences. Scientists of all disciplines use the ideas of physics, including chemists who study the structure of molecules, paleontologists who try to reconstruct how dinosaurs walked, and climatologists who study how human activities affect the atmosphere and oceans. Physics is also the foundation of all engineering and technology. No engineer could design a flat-screen TV, an interplanetary spacecraft, or even a better mousetrap without first understanding the basic laws of physics. (...) You will come to see physics as a towering achievement of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fick's Law Of Diffusion
Fick's laws of diffusion describe diffusion and were first posited by Adolf Fick in 1855 on the basis of largely experimental results. They can be used to solve for the Mass diffusivity, diffusion coefficient, . Fick's first law can be used to derive his second law which in turn is identical to the diffusion equation. ''Fick's first law'': Movement of particles from high to low concentration (diffusive flux) is directly proportional to the particle's concentration gradient. ''Fick's second law'': Prediction of change in concentration gradient with time due to diffusion. A diffusion process that obeys Fick's laws is called normal or Fickian diffusion; otherwise, it is called anomalous diffusion or non-Fickian diffusion. History In 1855, physiologist Adolf Fick first reported* * his now well-known laws governing the transport of mass through diffusive means. Fick's work was inspired by the earlier experiments of Thomas Graham (chemist), Thomas Graham, which fell short of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleation
In thermodynamics, nucleation is the first step in the formation of either a new Phase (matter), thermodynamic phase or Crystal structure, structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that determines how long an observer has to wait before the new phase or self-organized structure appears. For example, if a volume of water is cooled (at atmospheric pressure) significantly below 0°C, it will tend to Freezing, freeze into ice, but volumes of water cooled only a few degrees below 0°C often stay completely free of ice for long periods (supercooling). At these conditions, nucleation of ice is either slow or does not occur at all. However, at lower temperatures nucleation is fast, and ice crystals appear after little or no delay. Nucleation is a common mechanism which generates first-order phase transitions, and it is the start of the process of forming a new thermodynamic phase. In contrast, new phas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, , indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, is also called "water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
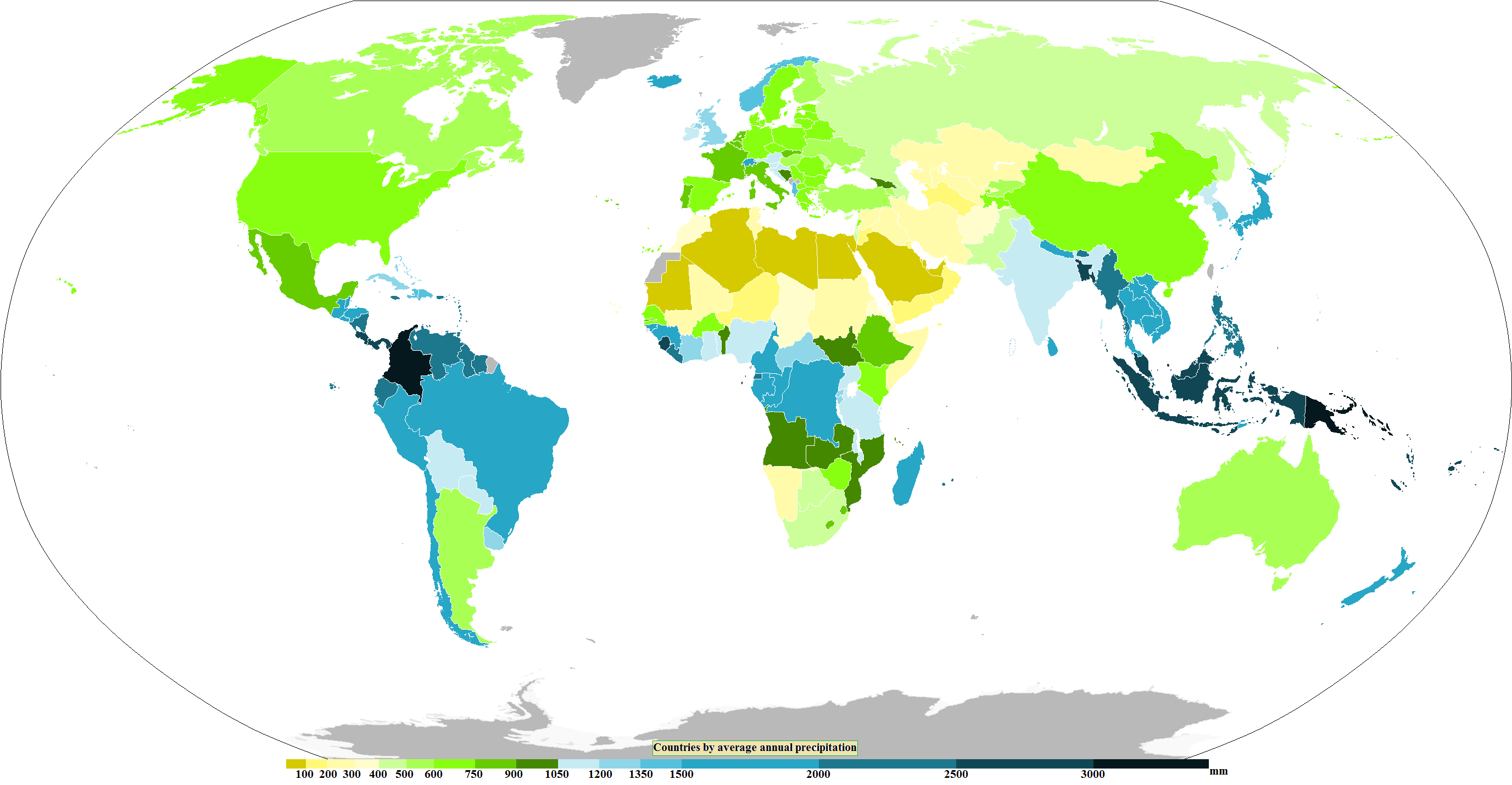
Precipitation (meteorology)
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwealth usage), snow, ice pellets, graupel and hail. Precipitation occurs when a portion of the atmosphere becomes saturated with water vapor (reaching 100% relative humidity), so that the water condenses and "precipitates" or falls. Thus, fog and mist are not precipitation; their water vapor does not condense sufficiently to precipitate, so fog and mist do not fall. (Such a non-precipitating combination is a colloid.) Two processes, possibly acting together, can lead to air becoming saturated with water vapor: cooling the air or adding water vapor to the air. Precipitation forms as smaller droplets coalesce via collision with other rain drops or ice crystals within a cloud. Short, intense periods of rain in scattered locations are called shower (p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophoresis
Electrophoresis is the motion of charged dispersed particles or dissolved charged molecules relative to a fluid under the influence of a spatially uniform electric field. As a rule, these are zwitterions with a positive or negative net charge. Electrophoresis is used in laboratories to separate macromolecules based on their charges. The technique normally applies a negative charge called cathode so anionic protein molecules move towards a positive charge called anode. Therefore, electrophoresis of positively charged particles or molecules (cations) is sometimes called cataphoresis, while electrophoresis of negatively charged particles or molecules (anions) is sometimes called anaphoresis. Electrophoresis is the basis for analytical techniques used in biochemistry and molecular biology to separate particles, molecules, or ions by size, charge, or binding affinity, either freely or through a supportive medium using a one-directional flow of electrical charge. It is use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffusiophoresis
Diffusiophoresis is the spontaneous motion of colloidal particles or molecules in a fluid, induced by a concentration gradient of a different substance. In other words, it is motion of one species, A, in response to a concentration gradient in another species, B. Typically, A is colloidal particles which are in aqueous solution in which B is a dissolved salt such as sodium chloride, and so the particles of A are much larger than the ions of B. But both A and B could be polymer molecules, and B could be a small molecule. For example, concentration gradients in ethanol solutions in water move 1 μm diameter colloidal particles with diffusiophoretic velocities _\text of order , the movement is towards regions of the solution with lower ethanol concentration (and so higher water concentration). Both species A and B will typically be diffusing but diffusiophoresis is distinct from simple diffusion: in simple diffusion a species A moves down a gradient in its own concentration. Diffus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Turbophoresis
Turbophoresis is the tendency for particles to migrate in the direction of decreasing turbulence level. The principle tends to segregate particles entrained in high velocity gases axially toward the wall region. Caporaloni et al. (1975) first found that because the vertical gradient of turbulence near a depositing surface is large, turbophoresis is expected to enhance rate of particle deposition onto the surface. It was also predicted independently by Reeks (1983) who derived it rigorously from the particle kinetic equation and showed that it arose from a force balance between the net drag force and the gradient of the particle kinetic stresses acting on the particles due to the turbulence. He predicted that this would lead to a buildup of concentration near the wall, a feature which has been observed both experimentally and in Direct Numerical Simulation A direct numerical simulation (DNS)https://eprints.soton.ac.uk/66182/1/A_primer_on_DNS.pdf "A Primer on Direct Numeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermophoresis
Thermophoresis (also thermomigration, thermodiffusion, the Soret effect, or the Ludwig–Soret effect) is a phenomenon observed in mixtures of mobile particles where the different particle types exhibit different responses to the force of a temperature gradient. This phenomenon tends to move light molecules to hot regions and heavy molecules to cold regions. The term ''thermophoresis'' most often applies to aerosol mixtures whose mean free path \lambda is comparable to its characteristic length scale L, but may also commonly refer to the phenomenon in all phases of matter. The term ''Soret effect'' normally applies to liquid mixtures, which behave according to different, less well-understood mechanisms than gaseous mixtures. Thermophoresis may not apply to thermomigration in solids, especially multi-phase alloys. Thermophoretic force The phenomenon is observed at the scale of one millimeter or less. An example that may be observed by the naked eye with good lighting is when the h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eddy (fluid Dynamics)
In fluid dynamics, an eddy is the swirling of a fluid and the reverse current (water), current created when the fluid is in a Turbulence, turbulent flow regime. The moving fluid creates a space devoid of downstream-flowing fluid on the downstream side of the object. Fluid behind the obstacle flows into the void creating a swirl of fluid on each edge of the obstacle, followed by a short reverse flow of fluid behind the obstacle flowing upstream, toward the back of the obstacle. This phenomenon is naturally observed behind large emergent rocks in swift-flowing rivers. An eddy is a movement of fluid that deviates from the general flow of the fluid. An example for an eddy is a vortex which produces such deviation. However, there are other types of eddies that are not simple vortices. For example, a Rossby wave is an eddy which is an undulation that is a deviation from mean flow, but does not have the local closed streamlines of a vortex. Swirl and eddies in engineering The propensi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Turbulence
In fluid dynamics, turbulence or turbulent flow is fluid motion characterized by chaotic changes in pressure and flow velocity. It is in contrast to laminar flow, which occurs when a fluid flows in parallel layers with no disruption between those layers. Turbulence is commonly observed in everyday phenomena such as surf, fast flowing rivers, billowing storm clouds, or smoke from a chimney, and most fluid flows occurring in nature or created in engineering applications are turbulent. Turbulence is caused by excessive kinetic energy in parts of a fluid flow, which overcomes the damping effect of the fluid's viscosity. For this reason, turbulence is commonly realized in low viscosity fluids. In general terms, in turbulent flow, unsteady vortices appear of many sizes which interact with each other, consequently drag due to friction effects increases. The onset of turbulence can be predicted by the dimensionless Reynolds number, the ratio of kinetic energy to viscous damping ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aerosol Impaction
In the physics of aerosols, aerosol impaction is the process in which particles are removed from an air stream by forcing the gases to make a sharp bend. Particles above a certain size possess so much momentum that they can not follow the air stream and strike a collection surface, which is available for later analysis of mass and composition. Removal of particles from an air-stream by impaction followed by mass and composition analysis has always been a different approach as to filter sampling, yet has been little utilized for routine analysis because of lack of suitable analytical methods. Advantages The most clear and important advantage of impaction, as opposed to filtration, is that two key aerosol parameters, size and composition, can be simultaneously established. There are many advantages of imp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |