|
Colloid Mill
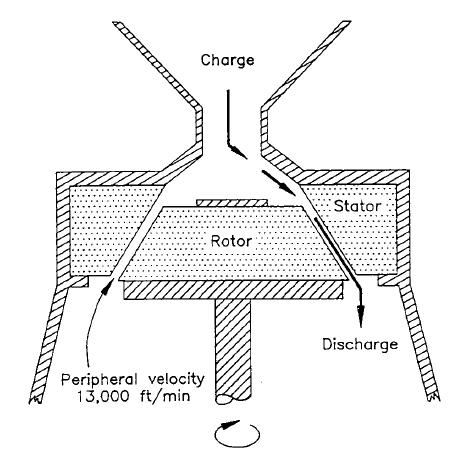
A colloid mill is a machine that is used to reduce the particle size of a solid in suspension in a liquid, or to reduce the droplet size in emulsions. Colloid mills work on the rotor-stator principle: a rotor turns at high speeds (2000 - 18000 RPM). A high level of stress is applied on the fluid which results in disrupting and breaking down the structure. Colloid mills are frequently used to increase the stability of suspensions and emulsion An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Altho ...s, but can also be used to reduce the particle size of solids in suspensions. Higher shear rates lead to smaller droplets, down to approximately 1 μm which are more resistant to emulsion separation. Application suitability Colloid mills are used in the following industries: * Pharmaceutic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid
Solid is one of the four fundamental states of matter (the others being liquid, gas, and plasma). The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural rigidity and resistance to a force applied to the surface. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas. The atoms in a solid are bound to each other, either in a regular geometric lattice ( crystalline solids, which include metals and ordinary ice), or irregularly (an amorphous solid such as common window glass). Solids cannot be compressed with little pressure whereas gases can be compressed with little pressure because the molecules in a gas are loosely packed. The branch of physics that deals with solids is called solid-state physics, and is the main branch of condensed matter physics (which also includes liquids). Materials s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Suspension (chemistry)
In chemistry, a suspension is a heterogeneous mixture of a fluid that contains solid particles sufficiently large for sedimentation. The particles may be visible to the naked eye, usually must be larger than one micrometer, and will eventually settle, although the mixture is only classified as a suspension when and while the particles have not settled out. Properties A suspension is a heterogeneous mixture in which the solute particles do not dissolve, but get suspended throughout the bulk of the solvent, left floating around freely in the medium. The internal phase (solid) is dispersed throughout the external phase (fluid) through mechanical agitation, with the use of certain excipients or suspending agents. An example of a suspension would be sand in water. The suspended particles are visible under a microscope and will settle over time if left undisturbed. This distinguishes a suspension from a colloid, in which the colloid particles are smaller and do not settle. Col ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, and plasma), and is the only state with a definite volume but no fixed shape. A liquid is made up of tiny vibrating particles of matter, such as atoms, held together by intermolecular bonds. Like a gas, a liquid is able to flow and take the shape of a container. Most liquids resist compression, although others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly constant density. A distinctive property of the liquid state is surface tension, leading to wetting phenomena. Water is by far the most common liquid on Earth. The density of a liquid is usually close to that of a solid, and much higher than that of a gas. Therefore, liquid and solid are both termed condensed mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Revolutions Per Minute
Revolutions per minute (abbreviated rpm, RPM, rev/min, r/min, or with the notation min−1) is a unit of rotational speed or rotational frequency for rotating machines. Standards ISO 80000-3:2019 defines a unit of rotation as the dimensionless unit equal to 1, which it refers to as a revolution, but does not define the revolution as a unit. It defines a unit of rotational frequency equal to s−1. The superseded standard ISO 80000-3:2006 did however state with reference to the unit name 'one', symbol '1', that "The special name revolution, symbol r, for this unit is widely used in specifications on rotating machines." The International System of Units (SI) does not recognize rpm as a unit, and defines the unit of frequency, Hz, as equal to s−1. :\begin 1~&\text &&=& 60~&\text \\ \frac~&\text &&=& 1~&\text \end A corresponding but distinct quantity for describing rotation is angular velocity, for which the SI unit is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydraulic
Hydraulics (from Greek: Υδραυλική) is a technology and applied science using engineering, chemistry, and other sciences involving the mechanical properties and use of liquids. At a very basic level, hydraulics is the liquid counterpart of pneumatics, which concerns gases. Fluid mechanics provides the theoretical foundation for hydraulics, which focuses on the applied engineering using the properties of fluids. In its fluid power applications, hydraulics is used for the generation, control, and transmission of power by the use of pressurized liquids. Hydraulic topics range through some parts of science and most of engineering modules, and cover concepts such as pipe flow, dam design, fluidics and fluid control circuitry. The principles of hydraulics are in use naturally in the human body within the vascular system and erectile tissue. Free surface hydraulics is the branch of hydraulics dealing with free surface flow, such as occurring in rivers, canals, lakes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stress (physics)
In continuum mechanics, stress is a physical quantity. It is a quantity that describes the magnitude of forces that cause deformation. Stress is defined as ''force per unit area''. When an object is pulled apart by a force it will cause elongation which is also known as deformation, like the stretching of an elastic band, it is called tensile stress. But, when the forces result in the compression of an object, it is called compressive stress. It results when forces like tension or compression act on a body. The greater this force and the smaller the cross-sectional area of the body on which it acts, the greater the stress. Therefore, stress is measured in newton per square meter (N/m2) or pascal (Pa). Stress expresses the internal forces that neighbouring particles of a continuous material exert on each other, while strain is the measure of the deformation of the material. For example, when a solid vertical bar is supporting an overhead weight, each particle in the bar pushe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emulsion
An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms ''colloid'' and ''emulsion'' are sometimes used interchangeably, ''emulsion'' should be used when both phases, dispersed and continuous, are liquids. In an emulsion, one liquid (the dispersed phase) is dispersed in the other (the continuous phase). Examples of emulsions include vinaigrettes, homogenized milk, liquid biomolecular condensates, and some cutting fluids for metal working. Two liquids can form different types of emulsions. As an example, oil and water can form, first, an oil-in-water emulsion, in which the oil is the dispersed phase, and water is the continuous phase. Second, they can form a water-in-oil emulsion, in which water is the dispersed phase and oil is the continuous phase. Multiple emulsions are also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Micrometre
The micrometre ( international spelling as used by the International Bureau of Weights and Measures; SI symbol: μm) or micrometer (American spelling), also commonly known as a micron, is a unit of length in the International System of Units (SI) equalling (SI standard prefix "micro-" = ); that is, one millionth of a metre (or one thousandth of a millimetre, , or about ). The nearest smaller common SI unit is the nanometre, equivalent to one thousandth of a micrometre, one millionth of a millimetre or one billionth of a metre (). The micrometre is a common unit of measurement for wavelengths of infrared radiation as well as sizes of biological cells and bacteria, and for grading wool by the diameter of the fibres. The width of a single human hair ranges from approximately 20 to . The longest human chromosome, chromosome 1, is approximately in length. Examples Between 1 μm and 10 μm: * 1–10 μm – length of a typical bacterium * 3–8 μm – width of st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schematic Diagram Of Colloid Mill
A schematic, or schematic diagram, is a designed representation of the elements of a system using abstract, graphic symbols rather than realistic pictures. A schematic usually omits all details that are not relevant to the key information the schematic is intended to convey, and may include oversimplified elements in order to make this essential meaning easier to grasp, as well as additional organization of the information. For example, a subway map intended for passengers may represent a subway station with a dot. The dot is not intended to resemble the actual station at all but aims to give the viewer information without unnecessary visual clutter. A schematic diagram of a chemical process uses symbols in place of detailed representations of the vessels, piping, valves, pumps, and other equipment that compose the system, thus emphasizing the functions of the individual elements and the interconnections among them and suppresses their physical details. In an electronic circuit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homogenization (chemistry)
Homogenization or homogenisation is any of several processes used to make a mixture of two mutually non-soluble liquids the same throughout. This is achieved by turning one of the liquids into a state consisting of extremely small particles distributed uniformly throughout the other liquid. A typical example is the homogenization of milk, wherein the milk fat globules are reduced in size and dispersed uniformly through the rest of the milk. Definition Homogenization (from "homogeneous;" Greek, ''homogenes'': ''homos,'' same + ''genos,'' kind) is the process of converting two immiscible liquids (i.e. liquids that are not soluble, in all proportions, one in another) into an emulsion (Mixture of two or more liquids that are generally immiscible). Sometimes two types of homogenization are distinguished: primary homogenization, when the emulsion is created directly from separate liquids; and secondary homogenization, when the emulsion is created by the reduction in size of droplets ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |