|
Cinanserin
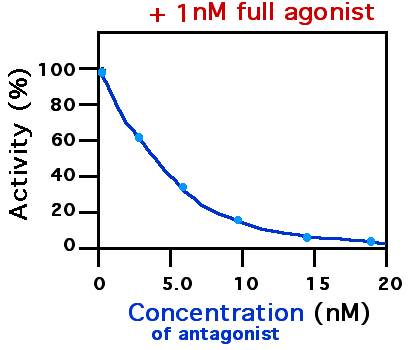
Cinanserin (INN) is a 5-HT2A and 5-HT2C receptor antagonist which was discovered in the 1960s. The molecule is an inhibitor of the 3C-like protease of SARS-coronavirus (SARS). See also * Sarpogrelate * Ketanserin Ketanserin ( INN, USAN, BAN) (brand name Sufrexal; former developmental code name R41468) is a drug used clinically as an antihypertensive agent and in scientific research to study the serotonin system; specifically, the 5-HT2 receptor family. I ... * Ritanserin References 5-HT2A antagonists 5-HT2C antagonists Thioethers Anilides Abandoned drugs {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2C Receptor
The 5-HT2C receptor is a subtype of 5-HT receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). It is a G protein-coupled receptor (GPCR) that is coupled to Gq/G11 and mediates excitatory neurotransmission. ''HTR2C'' denotes the human gene encoding for the receptor, that in humans is located at the X chromosome. As males have one copy of the gene and in females one of the two copies of the gene is repressed, polymorphisms at this receptor can affect the two sexes to differing extent. Structure At the cell surface the receptor exists as a homodimer. The crystal structure is known since 2018. Distribution 5-HT2C receptors are located mainly in the choroid plexus, and in rats is also found in many other brain regions in high concentrations, including parts of the hippocampus, anterior olfactory nucleus, substantia nigra, several brainstem nuclei, amygdala, subthalamic nucleus and lateral habenula. 5-HT2C receptors are also found on epithel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sarpogrelate
Sarpogrelate (former developmental code names MCI-9042, LS-187,118) is a drug which acts as an antagonist at the 5HT2A and 5-HT2B receptors. It blocks serotonin-induced platelet aggregation, and has applications in the treatment of many diseases including diabetes mellitus, Buerger's disease, Raynaud's disease, coronary artery disease, angina pectoris, and atherosclerosis. See also * Cinanserin * Naftidrofuryl Naftidrofuryl (International Nonproprietary Name, INN), also known as nafronyl or as the oxalate salt naftidrofuryl oxalate or nafronyl oxalate, is a vasodilator used in the management of peripheral and Cerebrum, cerebral Blood vessel, vascular d ... References {{Serotonergics 5-HT2 antagonists Phenol ethers Carboxylate esters Dimethylamino compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2A Receptor
The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations. 5-HT is short for 5-hydroxy-tryptamine or serotonin. This is the main excitatory receptor subtype among the GPCRs for serotonin, although 5-HT2A may also have an inhibitory effect on certain areas such as the visual cortex and the orbitofrontal cortex. This receptor was first noted for its importance as a target of serotonergic psychedelic drugs such as LSD and psilocybin mushrooms. Later it came back to prominence because it was also found to be mediating, at least partly, the action of many antipsychotic drugs, especially the atypical ones. Downregulation of post-synaptic 5-HT2A receptor is an adaptive process provoked by chronic administration of selective serotonin reuptake inhibitors (SSRIs) and atypical antipsychotics. Suicidal and otherwis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves " '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, |
SARS
Severe acute respiratory syndrome (SARS) is a viral respiratory disease of zoonotic origin caused by the severe acute respiratory syndrome coronavirus (SARS-CoV or SARS-CoV-1), the first identified strain of the SARS coronavirus species, ''severe acute respiratory syndrome–related coronavirus'' (SARSr-CoV). The first known cases occurred in November 2002, and the syndrome caused the 2002–2004 SARS outbreak. In the 2010s, Chinese scientists traced the virus through the intermediary of Asian palm civets to cave-dwelling horseshoe bats in Xiyang Yi Ethnic Township, Yunnan.The locality was referred to be "a cave in Kunming" in earlier sources because the Xiyang Yi Ethnic Township is administratively part of Kunming, though 70 km apart. Xiyang was identified on * For an earlier interview of the researchers about the locality of the caves, see: SARS was a relatively rare disease; at the end of the epidemic in June 2003, the incidence was 8,469 cases with a case fatality rate (CFR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketanserin
Ketanserin (INN, USAN, BAN) (brand name Sufrexal; former developmental code name R41468) is a drug used clinically as an antihypertensive agent and in scientific research to study the serotonin system; specifically, the 5-HT2 receptor family. It was discovered at Janssen Pharmaceutica in 1980. It is not available in the United States. Uses Medical uses Ketanserin is classified as an antihypertensive by the World Health Organization and the National Institute of Health. It has been used to reverse pulmonary hypertension caused by protamine (which in turn was administered to reverse the effects of heparin overdose). The reduction in hypertension is not associated with reflex tachycardia. It has been used in cardiac surgery. A 2000 Cochrane Review found that, compared to placebo, ketanserin did not provide significant relief for people suffering from Raynaud's phenomenon attacks in the setting of progressive systemic sclerosis (an autoimmune disorder). While the frequency of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ritanserin
Ritanserin, also known by its developmental code name R-55667, is a serotonin antagonist medication described as an anxiolytic, antidepressant, antiparkinsonian agent, and antihypertensive agent. It was never marketed for medical use due to safety problems but has been used in scientific research to study the serotonin system. Pharmacology Pharmacodynamics Ritanserin acts as a selective 5-HT2A (Ki = 0.45 nM) and 5-HT2C receptor (Ki = 0.71 nM) antagonist. It has relatively low affinity for the H1, D2, α1-adrenergic, and α2-adrenergic receptors (39-, 77-, 107-, and 166-fold lower relative to 5-HT2A, respectively). The affinity of ritanserin for the 5-HT1A receptor is less than 1 μM. In addition to its affinity for the 5-HT2A and 5-HT2C receptors, ritanserin also binds to and antagonizes the 5-HT1D, 5-HT2B, 5-HT5A, 5-HT6, and 5-HT7 receptors. History The atypical antipsychotic risperidone was developed from ritanserin. Society and culture Names ''Ritanserin'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2A Antagonists
The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations. 5-HT is short for 5-hydroxy-tryptamine or serotonin. This is the main excitatory receptor subtype among the GPCRs for serotonin, although 5-HT2A may also have an inhibitory effect on certain areas such as the visual cortex and the orbitofrontal cortex. This receptor was first noted for its importance as a target of serotonergic psychedelic drugs such as LSD and psilocybin mushrooms. Later it came back to prominence because it was also found to be mediating, at least partly, the action of many antipsychotic drugs, especially the atypical ones. Downregulation of post-synaptic 5-HT2A receptor is an adaptive process provoked by chronic administration of selective serotonin reuptake inhibitors (SSRIs) and atypical antipsychotics. Suicidal and otherwise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2C Antagonists
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction. Approximately 90% of the serotonin that the body produces is in the intestinal tract. Biochemically, the indoleamine molecule derives from the amino acid tryptophan, via the (rate-limiting) hydroxylation of the 5 position on the ring (forming the intermediate 5-hydroxytryptophan), and then decarboxylation to produce serotonin. Serotonin is primarily found in the enteric nervous system located in the gastrointestinal tract (GI tract). However, it is also produced in the central nervous system (CNS), specifically in the raphe nuclei located in the brainstem, Merkel cells located in the skin, pulmonary neuroendocrine cells and taste receptor cells in the tongue. Additionally, serotonin is stored in blood platelets and is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioethers
In organic chemistry, an organic sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application. Nomenclature Sulfides are sometimes called thioethers, especially in the old literature. The two organic substituents are indicated by the prefixes. (CH3)2S is called dimethylsulfide. Some sulfides are named by modifying the common name for the corresponding ether. For example, C6H5SCH3 is methyl phenyl sulfide, but is more commonly called thioanisole, since its structure is related to that for anisole, C6H5OCH3. The modern s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anilides
Anilides (or phenylamides) are a class of chemical compounds, which are amide derivatives of aniline. Preparation Aniline reacts with acyl chlorides or carboxylic anhydrides to give anilides. For example, reaction of aniline with acetyl chloride provides acetanilide (CH3-CO-NH-C6H5). At high temperatures, aniline and carboxylic acids react to give anilides. Uses * Herbicides * Fungicides - Oxycarboxin, Carboxin {{Short pages monitor [Baidu] |