|
Carbonate Ester
In organic chemistry, a carbonate ester (organic carbonate or organocarbonate) is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is and they are related to esters (), ethers () and also to the inorganic carbonates. Monomers of polycarbonate (e.g. Makrolon or Lexan) are linked by carbonate groups. These polycarbonates are used in eyeglass lenses, compact discs, and bulletproof glass. Small carbonate esters like dimethyl carbonate, ethylene carbonate, propylene carbonate are used as solvents, dimethyl carbonate is also a mild methylating agent. Structures Carbonate esters have planar OC(OC)2 cores, which confers rigidity. The unique O=C bond is short (1.173 Å in the depicted example), while the C-O bonds are more ether-like (the bond distances of 1.326 Å for the example depicted). Carbonate esters can be divided into three structural classes: acyclic, cyclic, and polymeri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate Ester
In organic chemistry, a carbonate ester (organic carbonate or organocarbonate) is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is and they are related to esters (), ethers () and also to the inorganic carbonates. Monomers of polycarbonate (e.g. Makrolon or Lexan) are linked by carbonate groups. These polycarbonates are used in eyeglass lenses, compact discs, and bulletproof glass. Small carbonate esters like dimethyl carbonate, ethylene carbonate, propylene carbonate are used as solvents, dimethyl carbonate is also a mild methylating agent. Structures Carbonate esters have planar OC(OC)2 cores, which confers rigidity. The unique O=C bond is short (1.173 Å in the depicted example), while the C-O bonds are more ether-like (the bond distances of 1.326 Å for the example depicted). Carbonate esters can be divided into three structural classes: acyclic, cyclic, and polymeri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
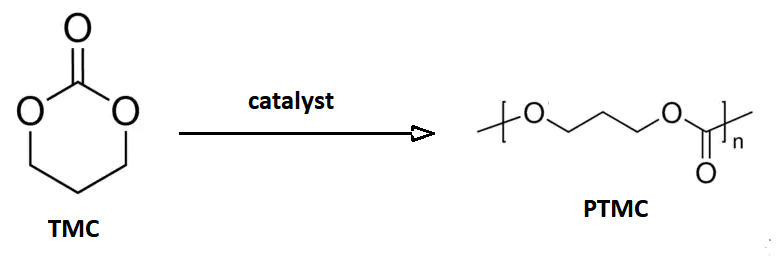
Trimethylene Carbonate
Trimethylene carbonate, or 1,3-propylene carbonate, is a 6-membered cyclic carbonate ester. It is a colourless solid that upon heating or catalytic ring-opening converts to poly(trimethylene carbonate) (PTMC). Such polymers are called aliphatic polycarbonates and are of interest for potential biomedical applications. An isomeric derivative is propylene carbonate, a colourless liquid that does not spontaneously polymerize. Preparation This compound may be prepared from 1,3-propanediol and ethyl chloroformate (a phosgene substitute), or from oxetane and carbon dioxide with an appropriate catalyst: :HOC3H6OH + ClCO2C2H5 → C3H6O2CO + C2H5OH + HCl :C3H6O + CO2 → C3H6O2CO This cyclic carbonate undergoes ring-opening polymerization to give poly(trimethylene carbonate), abbreviated PTMC. : Medical devices The polymer PTC is of commercial interest as a biodegradable polymer with biomedical applications. A block copolymer of glycolic acid Glycolic acid (or hydroxyacetic acid; ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Poly(propylene Carbonate)
Polypropylene carbonate (PPC), a copolymer of carbon dioxide and propylene oxide, is a thermoplastic material. Catalysts like zinc glutarate are used in polymerization. Properties Polypropylene carbonate is soluble in polar solvents like lower ketones, ethyl acetate, dichloromethane and chlorinated hydrocarbons and insoluble in solvents like alcohols, water, and aliphatic hydrocarbons. It also forms stable emulsions in water. PPC allows the diffusion of gases like oxygen through it. Having a glass temperature (Tg) between 25 and 45 °C, PPC binders are amorphous. The glass temperature of PPC is slightly greater than polyethylene carbonate (PEC). Its refractive index is 1.46 while its dielectric constant is 3. Applications Polypropylene carbonate is used to increase the toughness of some epoxy resins. It is used as a sacrificial binder in the ceramic industry, which decomposes and evaporates during sintering. It has a low sodium content which makes it suitable for the p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylene Carbonate
Trimethylene carbonate, or 1,3-propylene carbonate, is a 6-membered cyclic carbonate ester. It is a colourless solid that upon heating or catalytic ring-opening converts to poly(trimethylene carbonate) (PTMC). Such polymers are called aliphatic polycarbonates and are of interest for potential biomedical applications. An isomeric derivative is propylene carbonate, a colourless liquid that does not spontaneously polymerize. Preparation This compound may be prepared from 1,3-propanediol and ethyl chloroformate (a phosgene substitute), or from oxetane and carbon dioxide with an appropriate catalyst: :HOC3H6OH + ClCO2C2H5 → C3H6O2CO + C2H5OH + HCl :C3H6O + CO2 → C3H6O2CO This cyclic carbonate undergoes ring-opening polymerization to give poly(trimethylene carbonate), abbreviated PTMC. : Medical devices The polymer PTC is of commercial interest as a biodegradable polymer with biomedical applications. A block copolymer of glycolic acid Glycolic acid (or hydroxyacetic acid; ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Carbonate
Ethylene carbonate (sometimes abbreviated EC) is the organic compound with the formula (CH2O)2CO. It is classified as the cyclic carbonate ester of ethylene glycol and carbonic acid. At room temperature (25 °C) ethylene carbonate is a transparent crystalline solid, practically odorless and colorless, and somewhat soluble in water. In the liquid state (m.p. 34-37 °C) it is a colorless odorless liquid. JEFFSOL ETHYLENE CARBONATE catalog entry at www.huntsman.com. Accessed on 2010-02-18. Production and reactions Ethylene carbonate is produced by the reaction between and |
Dimethyl Dicarbonate
Dimethyl dicarbonate (DMDC) is a colorless liquid and a pungent odor at high concentration at room temperature. It is primarily used as a beverage preservative, processing aid, or sterilant ( INS No. 242) being highly active against typical beverage spoiling microorganisms like yeast, bacteria, or mould. Usage Dimethyl dicarbonate is used to stabilize beverages by preventing microbial spoilage. It can be used in various non-alcoholic as well as alcoholic drinks like wine, cider, beer-mix beverages or hard seltzers. Beverage spoiling microbes are killed by methoxycarbonylation of proteins. It acts by inhibiting enzymes involved in the microbial metabolism, e.g. acetate kinase and L-glutamic acid decarboxylase. It has also been proposed that DMDC inhibits the enzymes alcohol dehydrogenase and glyceraldehyde 3-phosphate dehydrogenase by causing the methoxycarbonylation of their histidine components. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ice ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, volatile, colourless, flammable liquid with a distinctive alcoholic odour similar to that of ethanol (potable alcohol). A polar solvent, methanol acquired the name wood alcohol because it was once produced chiefly by the destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl benzoate, anisole, peroxyacids, as well as a host of more specialised chemicals. Occurrence Small amounts of methanol are present in normal, healthy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to liquid water when in contact with a liquid or solid surface or cloud condensation nuclei within the atmosphere. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition. Initiation Condensation is initiated by the formation of atomic/molecular clusters of that species within its gaseous volume—like rain drop or snow flake formation within clouds—or at the contact between such gaseous phase and a liquid or solid surface. In clouds, this can be catalyzed by water-nucleating proteins, produced by atmospheric microbes, which are capable of binding gaseous or liquid water molecules. Reversibility scenarios A few distinct reversibility scenarios emerge here with respect to t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transesterification
In organic chemistry, transesterification is the process of exchanging the organic group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. The reaction can also be accomplished with the help of other enzymes, particularly lipases (one example is the lipase E.C.3.1.1.3). Strong acids catalyse the reaction by donating a proton to the carbonyl group, thus making it a more potent electrophile, whereas bases catalyse the reaction by removing a proton from the alcohol, thus making it more nucleophilic. If the alcohol produced by the reaction can be separated from the reactants by distillation this will drive the equilibrium toward the products, this means that esters with larger alkoxy groups can be made from methyl or ethyl esters in high purity by heating the mixture of ester, acid/base, and large alcohol. Mechanism In the transesterification mechanism, the carbonyl carbon of the startin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Carbonylation
Oxidative carbonylation is a class of reactions that use carbon monoxide in combination with an oxidant to generate esters and carbonate esters. These transformations utilize transition metal complexes as homogeneous catalysts. Many of these reactions employ palladium catalysts. Mechanistically, these reactions resemble the Wacker process. Illustrative oxidative carbonylations Oxidative carbonylation, using palladium-based catalysts, allows certain alkenes to be converted into homologated esters: :2 RCH=CH2 + 2 CO + O2 + 2 MeOH → 2 RCH=CHCO2Me + 2 H2O Such reactions are assumed to proceed by the insertion of the alkene into the Pd(II)-CO2Me bond of a metallacarboxylic ester followed by beta-hydride elimination (Me = CH3). Arylboronic acids react with Pd(II) compounds to give Pd(II)-aryl species, which undergo carbonylation to give Pd(II)-C(O)aryl. These benzyl-Pd intermediates are intercepted by alkenes, which insert. Subsequent beta-hydride elimination gives the arylket ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds, when insufficient oxygen or heat is present to produce carbon dioxide. There are also numerous environmental and biological sources that generate and emit a significant amount of carbon monoxide. It is important in the production of many compounds, including drugs, fragrances, and fuels. Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change. Carbon monoxide has important biological roles across phylogenetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |