|
Zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyish-white color that closely resembles hafnium and, to a lesser extent, titanium. It is solid at room temperature, Ductility, ductile, malleable and corrosion-resistant. The name ''zirconium'' is derived from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian Language, Persian ''Jargoon, zargun'' (zircon; ''zar-gun'', "gold-like" or "as gold"). Besides zircon, zirconium occurs in over 140 other minerals, including baddeleyite and eudialyte; most zirconium is produced as a byproduct of minerals mined for titanium and tin. Zirconium forms a variety of inorganic chemistry, inorganic compounds, such as zirconium dioxide, and organometallic compounds, such as zirconocene dichloride. Five isotope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Zirconium
Naturally occurring zirconium (40Zr) is composed of four stable isotopes (of which one may in the future be found radioactive), and one very long-lived radioisotope (96Zr), a primordial nuclide that decays via double beta decay with an observed half-life of 2.34 × 1019 years; it can also undergo single beta decay, which is not yet observed, but the theoretically predicted value of t1/2 is 2.4 × 1020 years. The second most stable radioisotope is 93Zr, which has a half-life of 1.61 million years. Thirty other radioisotopes have been observed. All have half-lives less than a day except for 95Zr (64.02 days), 88Zr (83.4 days), and 89Zr (78.41 hours). The primary decay mode is electron capture for isotopes lighter than 92Zr, and the primary mode for heavier isotopes is beta decay. List of isotopes , -id=Zirconium-77 , 77Zr , style="text-align:right" , 40 , style="text-align:right" , 37 , 76.96608(43)# , 100# μs , , , 3/2−# , , , -id=Zirconium-78 , 78Zr , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
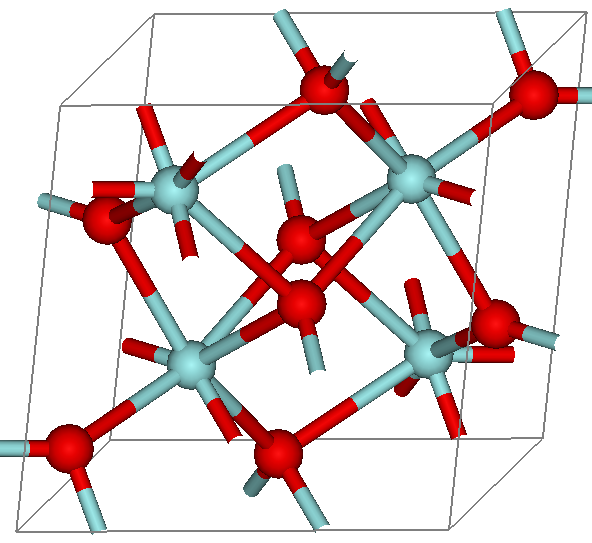
Zirconium Dioxide
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zirconium silicate or zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant stabilized cubic structured zirconia, cubic zirconia, is synthesized in various colours for use as a gemstone and a diamond simulant. Production, chemical properties, occurrence Zirconia is produced by calcining zirconium compounds, exploiting its high thermostability.Ralph Nielsen "Zirconium and Zirconium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. Structure Three phases are known: monoclinic below 1170 °C, tetragonal between 1170 °C and 2370 °C, and cubic above 2370 °C. The trend is for higher symmetry at higher temperatures, as is usually the case. A small percentage of the oxides of calcium or yttrium stabilize in the cubic phase. The very r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1922, by Dirk Coster and George de Hevesy. Hafnium is named after , the Latin name for Copenhagen, where it was discovered. Hafnium is used in filaments and electrodes. Some semiconductor fabrication processes use its oxide for integrated circuits at 45 nanometers and smaller feature lengths. Some superalloys used for special applications contain hafnium in combination with niobium, titanium, or tungsten. Hafnium's large neutron capture cross section makes it a good material for neutron absorption in control rods in nuclear power plants, but at the same time requires that it be removed from the neutron-transparent corrosion-resistant zirconium alloys used in nuclear r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zircon
Zircon () is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is zirconium(IV) silicate, and its corresponding chemical formula is Zr SiO4. An empirical formula showing some of the range of substitution in zircon is (Zr1–y, REEy)(SiO4)1–x(OH)4x–y. Zircon precipitates from silicate melts and has relatively high concentrations of high field strength incompatible elements. For example, hafnium is almost always present in quantities ranging from 1 to 4%. The crystal structure of zircon is tetragonal crystal system. The natural color of zircon varies between colorless, yellow-golden, red, brown, blue, and green. The name derives from the Persian ''zargun'', meaning "gold-hued". This word is changed into " jargoon", a term applied to light-colored zircons. The English word "zircon" is derived from ''Zirkon'', which is the German adaptation of this word. Yellow, orange, and red zircon is also known as " hyacint ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zirconocene Dichloride
Zirconocene dichloride is an organozirconium compound composed of a zirconium central atom, with two cyclopentadienyl and two chloro ligands. It is a colourless diamagnetic solid that is somewhat stable in air. Preparation and structure Zirconocene dichloride may be prepared from zirconium(IV) chloride-tetrahydrofuran complex and sodium cyclopentadienide: :ZrCl4(THF)2 + 2 NaCp → Cp2ZrCl2 + 2 NaCl + 2 THF The closely related compound Cp2ZrBr2 was first described by Birmingham and Wilkinson. The compound is a bent metallocene: the Cp rings are not parallel, the average Cp(centroid)-M-Cp angle being 128°. The Cl-Zr-Cl angle of 97.1° is wider than in niobocene dichloride (85.6°) and molybdocene dichloride (82°). This trend helped to establish the orientation of the HOMO in this class of complex. Reactions Schwartz's reagent Zirconocene dichloride reacts with lithium aluminium hydride to give Cp2ZrHCl Schwartz's reagent: :(C5H5)2ZrCl2 + 1/4 LiAlH4 → (C5H5)2ZrHCl + 1/4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baddeleyite
Baddeleyite is a rare zirconium oxide mineral (ZrO2 or zirconia), occurring in a variety of monoclinic prismatic crystal forms. It is transparent to translucent, has high Index of refraction, indices of refraction, and ranges from colorless to yellow, green, and dark brown. See etymology Baddeleyite#Origin of the name, below. Baddeleyite is a refractory mineral, with a melting point of 2700 °C. Hafnium is a substituting ''impurity'' and may be present in quantities ranging from 0.1 to several percent. It can be found in igneous rocks containing potassium feldspar and plagioclase. Baddeleyite is commonly not found with zircon (ZrSiO4), because it forms in silica-undersaturated rocks, such as mafic rocks. This is because, when silica is free in the system (silica-saturated/oversaturated), zircon is the dominating phase, not baddeleyite. It belongs to the Monoclinic crystal system, monoclinic-prismatic class, of the P21/c crystal system. It has been used for geochronology. Geo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fuel Rod
Nuclear fuel refers to any substance, typically fissile material, which is used by nuclear power stations or other atomic nucleus, nuclear devices to generate energy. Oxide fuel For fission reactors, the fuel (typically based on uranium) is usually based on the metal oxide; the oxides are used rather than the metals themselves because the oxide melting point is much higher than that of the metal and because it cannot burn, being already in the oxidized state. Uranium dioxide Uranium dioxide is a black semiconductor, semiconducting solid. It can be made by heating uranyl nitrate to form . : This is then converted by heating with hydrogen to form UO2. It can be made from Enriched uranium, enriched uranium hexafluoride by reacting with ammonia to form a solid called ammonium diuranate, . This is then heated (Calcination, calcined) to form and U3O8 which is then converted by heating with hydrogen or ammonia to form UO2. The UO2 is mixed with an organic binder and pressed in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |