|
X-ray Computed Tomography
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ranging from 10 nanometers to 10 picometers, corresponding to frequencies in the range of 30 petahertz to 30 exahertz ( to ) and photon energies in the range of 100 eV to 100 keV, respectively. X-rays were discovered in 1895 by the German scientist Wilhelm Conrad Röntgen, who named it ''X-radiation'' to signify an unknown type of radiation.Novelline, Robert (1997). ''Squire's Fundamentals of Radiology''. Harvard University Press. 5th edition. . X-rays can penetrate many solid substances such as construction materials and living tissue, so X-ray radiography is widely used in medical diagnostics (e.g., checking for broken bones) and materials science (e.g., identification of some chemical elements and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Color X-ray Photogram
Color (or colour in Commonwealth English; see spelling differences) is the visual perception based on the electromagnetic spectrum. Though color is not an inherent property of matter, color perception is related to an object's light absorption, emission, reflection and transmission. For most humans, colors are perceived in the visible light spectrum with three types of cone cells ( trichromacy). Other animals may have a different number of cone cell types or have eyes sensitive to different wavelengths, such as bees that can distinguish ultraviolet, and thus have a different color sensitivity range. Animal perception of color originates from different light wavelength or spectral sensitivity in cone cell types, which is then processed by the brain. Colors have perceived properties such as hue, colorfulness (saturation), and luminance. Colors can also be additively mixed (commonly used for actual light) or subtractively mixed (commonly used for materials). If the colors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
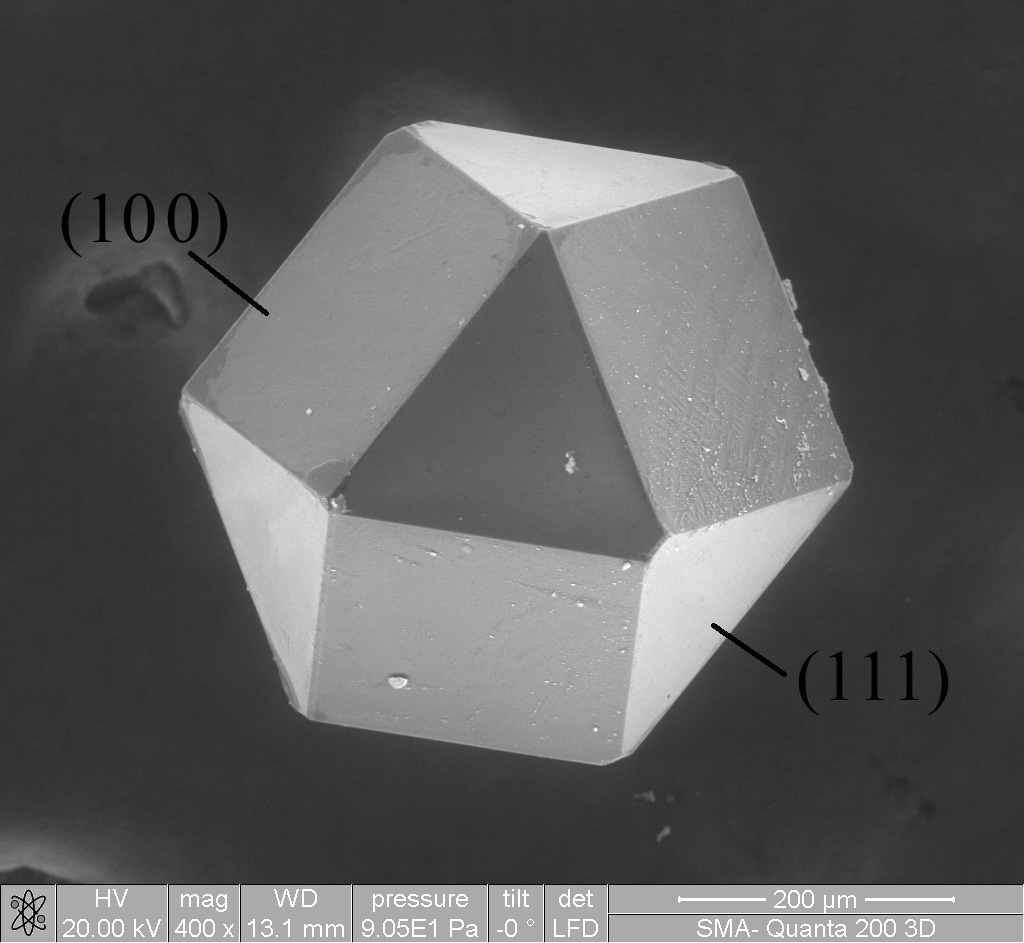
Materials Science
Materials science is an interdisciplinary field of researching and discovering materials. Materials engineering is an engineering field of finding uses for materials in other fields and industries. The intellectual origins of materials science stem from the Age of Enlightenment, when researchers began to use analytical thinking from chemistry, physics, and engineering to understand ancient, phenomenological observations in metallurgy and mineralogy. Materials science still incorporates elements of physics, chemistry, and engineering. As such, the field was long considered by academic institutions as a sub-field of these related fields. Beginning in the 1940s, materials science began to be more widely recognized as a specific and distinct field of science and engineering, and major technical universities around the world created dedicated schools for its study. Materials scientists emphasize understanding how the history of a material (''processing'') influences its struc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Voltage
Voltage, also known as (electrical) potential difference, electric pressure, or electric tension, is the difference in electric potential between two points. In a Electrostatics, static electric field, it corresponds to the Work (electrical), work needed per unit of Electric charge, charge to move a positive Test particle#Electrostatics, test charge from the first point to the second point. In the SI unit, International System of Units (SI), the SI derived unit, derived unit for voltage is the ''volt'' (''V''). The voltage between points can be caused by the build-up of electric charge (e.g., a capacitor), and from an electromotive force (e.g., electromagnetic induction in a Electric generator, generator). On a macroscopic scale, a potential difference can be caused by electrochemical processes (e.g., cells and batteries), the pressure-induced piezoelectric effect, and the thermoelectric effect. Since it is the difference in electric potential, it is a physical Scalar (physics ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Direct Current
Direct current (DC) is one-directional electric current, flow of electric charge. An electrochemical cell is a prime example of DC power. Direct current may flow through a conductor (material), conductor such as a wire, but can also flow through semiconductors, electrical insulation, insulators, or even through a vacuum as in electron beam, electron or ion beams. The electric current flows in a constant direction, distinguishing it from alternating current (AC). A archaism, term formerly used for this type of current was galvanic current. The abbreviations ''AC'' and ''DC'' are often used to mean simply ''alternating'' and ''direct'', as when they modify ''Electric current, current'' or ''voltage''. Direct current may be converted from an alternating current supply by use of a rectifier, which contains Electronics, electronic elements (usually) or electromechanical elements (historically) that allow current to flow only in one direction. Direct current may be converted into alt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionization
Ionization or ionisation is the process by which an atom or a molecule acquires a negative or positive Electric charge, charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules, electrons, positrons, protons, antiprotons, and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected. Uses Everyday examples of gas ionization occur within a fluorescent lamp or other electrical discharge lamps. It is also used in radiation detectors such as the Geiger- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1875 In Science
The year 1875 in science and technology involved some significant events, listed below. Chemistry * Gallium is discovered spectroscopically by French chemist Paul Emile Lecoq de Boisbaudran. Later in this year he obtains the free metal by electrolysis of its hydroxide and names it. This is the first of Dmitri Mendeleev's predicted elements to be identified. * Phenylhydrazine is discovered by Hermann Emil Fischer. * Swiss chocolatier Daniel Peter working with Henri Nestlé's company perfects a method of manufacturing milk chocolate using condensed milk. Earth sciences * March – ''Challenger'' expedition first records Challenger Deep. Genetics * Francis Galton publishes ''The History of Twins'', as a criterion of the relative powers of nature and nurture. Medicine * March 1 – The Hospital for Sick Children (Toronto) is founded in Canada. * The weekly medical journal, '' Deutsche Medizinische Wochenschrift'', is established in Germany by Paul Börner. Metrology * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crookes Tube
A Crookes tube: light and dark. Electrons (cathode rays) travel in straight lines from the cathode ''(left)'', as shown by the shadow cast by the metal Maltese cross on the fluorescence of the righthand glass wall of the tube. The anode is the electrode at the bottom A Crookes tube (also Crookes–Hittorf tube) is an early experimental discharge tube with partial vacuum invented by English physicist William Crookes and others around 1869–1875, in which cathode rays, streams of electrons, were discovered. Developed from the earlier Geissler tube, the Crookes tube consists of a partially evacuated glass bulb of various shapes, with two metal electrodes, the cathode and the anode, one at either end. When a high voltage is applied between the electrodes, cathode rays (electrons) are projected in straight lines from the cathode. It was used by Crookes, Johann Hittorf, Julius Plücker, Eugen Goldstein, Heinrich Hertz, Philipp Lenard, Kristian Birkeland and others to dis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up quark, up and down quark, down quarks. Electrons are extremely lightweight particles that orbit the positively charged atomic nucleus, nucleus of atoms. Their negative charge is balanced by the positive charge of protons in the nucleus, giving atoms their overall electric charge#Charge neutrality, neutral charge. Ordinary matter is composed of atoms, each consisting of a positively charged nucleus surrounded by a number of orbiting electrons equal to the number of protons. The configuration and energy levels of these orbiting electrons determine the chemical properties of an atom. Electrons are bound to the nucleus to different degrees. The outermost or valence electron, valence electrons are the least tightly bound and are responsible for th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathode Ray
Cathode rays are streams of electrons observed in discharge tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to electrons emitted from the cathode (the electrode connected to the negative terminal of the voltage supply). They were first observed in 1859 by German physicist Julius Plücker and Johann Wilhelm Hittorf, and were named in 1876 by Eugen Goldstein ''Kathodenstrahlen'', or cathode rays. In 1897, British physicist J. J. Thomson showed that cathode rays were composed of a previously unknown negatively charged particle, which was later named the ''electron''. Cathode-ray tubes (CRTs) use a focused beam of electrons deflected by electric or magnetic fields to render an image on a screen. Description Cathode rays are so named because they are emitted by the negative electrode, or cathode, in a vacuum tube. To release electrons into the tube, they first must be detach ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Discharge Tube
A gas-filled tube, also commonly known as a discharge tube or formerly as a Plücker tube, is an arrangement of electrodes in a gas within an insulating, temperature-resistant envelope. Gas-filled tubes exploit phenomena related to electric discharge in gases, and operate by ionizing the gas with an applied voltage sufficient to cause electrical conduction by the underlying phenomena of the Townsend discharge. A gas-discharge lamp is an electric light using a gas-filled tube; these include fluorescent lamps, metal-halide lamps, sodium-vapor lamps, and neon lights. Specialized gas-filled tubes such as krytrons, thyratrons, and ignitrons are used as switching devices in electric devices. The voltage required to initiate and sustain discharge is dependent on the pressure and composition of the fill gas and geometry of the tube. Although the envelope is typically glass, power tubes often use ceramics, and military tubes often use glass-lined metal. Both hot cathode an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiation
In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or a material medium. This includes: * ''electromagnetic radiation'' consisting of photons, such as radio waves, microwaves, infrared, visible light, ultraviolet, x-rays, and Gamma ray, gamma radiation (γ) * ''particle radiation'' consisting of particles of non-zero rest energy, such as alpha radiation (α), beta radiation (β), proton radiation and neutron radiation * ''acoustics, acoustic radiation'', such as ultrasound, sound, and seismic waves, all dependent on a physical transmission medium * ''gravitational radiation'', in the form of gravitational waves, ripples in spacetime Radiation is often categorized as either ''ionizing radiation, ionizing'' or ''non-ionizing radiation, non-ionizing'' depending on the energy of the radiated particles. Ionizing radiation carries more than 10 electron volt, electron volts (eV), which is enough to ionize atoms and molecul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |