|
Technetium Compounds
Technetium compounds are chemical compounds containing the chemical element technetium. Technetium can form multiple oxidation states, but often forms in the +4 and +7 oxidation states. Because technetium is radioactive, technetium compounds are extremely rare on Earth. Pertechnetate and derivatives The most prevalent form of technetium that is easily accessible is sodium pertechnetate, Na[TcO4]. The majority of this material is produced by radioactive decay from [99MoO4]2−: :[99MoO4]2− → [99mTcO4]− + e− Pertechnetate (tetroxidotechnetate) behaves analogously to perchlorate, both of which are tetrahedral molecular geometry, tetrahedral. Unlike permanganate (), it is only a weak oxidizing agent. Related to pertechnetate is Technetium(VII) oxide, technetium heptoxide. This pale-yellow, volatile solid is produced by oxidation of Tc metal and related precursors: :4 Tc + 7 O2 → 2 Tc2O7 It is a molecular metal oxide, analogous to manganese heptoxide. It adopts a Centro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its atomic nucleus, nucleus. Atoms of the same element can have different numbers of neutrons in their nuclei, known as isotopes of the element. Two or more atoms can combine to form molecules. Some elements form Homonuclear molecule, molecules of atoms of said element only: e.g. atoms of hydrogen (H) form Diatomic molecule, diatomic molecules (H). Chemical compounds are substances made of atoms of different elements; they can have molecular or non-molecular structure. Mixtures are materials containing different chemical substances; that means (in case of molecular substances) that they contain different types of molecules. Atoms of one element can be transformed into atoms of a different element in nuclear reactions, which change an atom's at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Telluride (chemistry)
The telluride ion is the anion Te2− and its derivatives. It is analogous to the other chalcogenide anions, the lighter O2−, S2−, and Se2−, and the heavier Po2−. In principle, Te2− is formed by the two-e− reduction of tellurium. The redox potential is −1.14 V. :Te(s) + 2 e− ↔ Te2− Although solutions of the telluride dianion have not been reported, soluble salts of bitelluride (TeH−) are known. Organic tellurides ''Tellurides'' also describe a class of organotellurium compounds formally derived from Te2−. An illustrative member is dimethyl telluride, which results from the methylation of telluride salts: :2 CH3I + Na2Te → (CH3)2Te + 2 NaI Dimethyl telluride is formed by the body when tellurium is ingested. Such compounds are often called telluroethers because they are structurally related to ethers with tellurium replacing oxygen, although the length of the C–Te bond is much longer than a C–O bond. C–Te–C angles tend to be c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name originates from Spanish language, Spanish , a diminutive of "silver". Platinum is a member of the platinum group of elements and group 10 element, group 10 of the periodic table of elements. It has six naturally occurring isotopes. It is one of the Abundance of elements in Earth's crust, rarer elements in Earth's crust, with an average abundance of approximately 5 microgram, μg/kg, making platinum about 30 times rarer than gold. It occurs in some nickel and copper ores along with some Native element mineral, native deposits, with 90% of current production from deposits across Russia's Ural Mountains, Colombia, the Sudbury Basin, Sudbury basin of Canada, and a large reserve in South Africa. Because of its scarcity in Earth's crust, only a f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1922, by Dirk Coster and George de Hevesy. Hafnium is named after , the Latin name for Copenhagen, where it was discovered. Hafnium is used in filaments and electrodes. Some semiconductor fabrication processes use its oxide for integrated circuits at 45 nanometers and smaller feature lengths. Some superalloys used for special applications contain hafnium in combination with niobium, titanium, or tungsten. Hafnium's large neutron capture cross section makes it a good material for neutron absorption in control rods in nuclear power plants, but at the same time requires that it be removed from the neutron-transparent corrosion-resistant zirconium alloys used in nuclear r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyish-white color that closely resembles hafnium and, to a lesser extent, titanium. It is solid at room temperature, Ductility, ductile, malleable and corrosion-resistant. The name ''zirconium'' is derived from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian Language, Persian ''Jargoon, zargun'' (zircon; ''zar-gun'', "gold-like" or "as gold"). Besides zircon, zirconium occurs in over 140 other minerals, including baddeleyite and eudialyte; most zirconium is produced as a byproduct of minerals mined for titanium and tin. Zirconium forms a variety of inorganic chemistry, inorganic compounds, such as zirconium dioxide, and organometallic compounds, such as zirconocene dichloride. Five isotope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation State
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. Beside nearly-pure ionic bonding, many covalent bonds exhibit a strong ionicity, making oxidation state a useful predictor of charge. The oxidation state of an atom does not represent the "real" charge on that atom, or any other actual atomic property. This is particularly true of high oxidation states, where the ionization energy required to produce a multiply positive ion is far greater than the energies available in chemical reactions. Additionally, the oxidation states of atoms in a given compound may vary depending on Electronegativities of the elements (data page), the choice of electronegativity scale used in their calculation. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TcCl4
Technetium(IV) chloride is the inorganic compound with the formula TcCl4. It was discovered in 1957 as the first binary halide of technetium. It is the highest oxidation binary chloride of technetium that has been isolated as a solid. It is volatile at elevated temperatures and its volatility has been used for separating technetium from other metal chlorides. Colloidal solutions of technetium(IV) chloride are oxidized to form Tc(VII) ions when exposed to gamma rays. Technetium tetrachloride can be synthesized from the reaction of Cl2 with technetium metal at elevated temperatures between 300 and 500 °C: :Tc + 2 Cl2 → TcCl4 Technetium tetrachloride has also been prepared from the reaction of technetium(VII) oxide with carbon tetrachloride in a sealed vessel at elevated temperature: :Tc2O7 + 7 CCl4 → 2 TcCl4 + 7 COCl2 + 3 Cl2 At 450 °C under vacuum, TcCl4 decomposes to TcCl3 and TcCl2. As verified by X-ray crystallography X-ray crystallography is the exper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TcF6
Technetium hexafluoride or technetium(VI) fluoride ( Tc F6) is a yellow inorganic compound with a low melting point. It was first identified in 1961. In this compound, technetium has an oxidation state of +6, the highest oxidation state found in the technetium halides. In this respect, technetium differs from rhenium, which forms a heptafluoride, ReF7. Technetium hexafluoride occurs as an impurity in uranium hexafluoride, as technetium is a fission product of uranium (spontaneous fission in natural uranium, possible contamination from induced fission inside the reactor in reprocessed uranium). The fact that the boiling point of the hexafluorides of uranium and technetium are very close to each other presents a problem in using fluoride volatility in nuclear reprocessing. Preparation Technetium hexafluoride is prepared by heating technetium metal with an excess of F2 at 400 °C. :Tc + 3 → Description Technetium hexafluoride is a golden-yellow solid at room tempera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
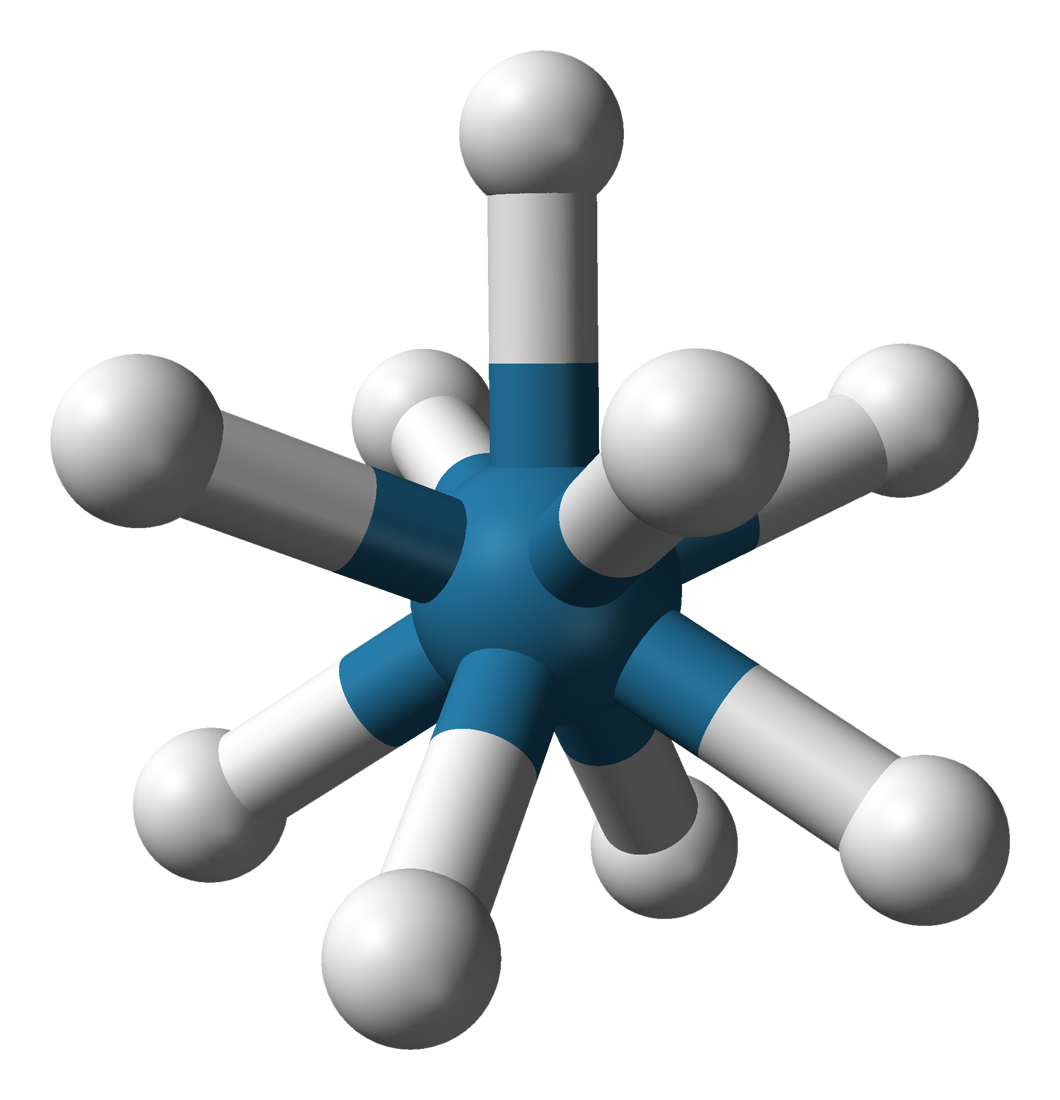
Potassium Nonahydridorhenate
Potassium nonahydridorhenate(VII) is an inorganic compound having the formula . This colourless salt is soluble in water but only poorly soluble in most alcohols. This salt contains the nonahydridorhenate(VII) anion, , which is a rare example of a coordination complex bearing only hydride ligands. History The study of rhenium hydrides can be traced to the 1950s and included reports of the "rhenide" anion, supposedly . These reports led to a series of investigations by A. P. Ginsberg and coworkers on the products from the reduction of perrhenate. The ''rhenide'' anion, , was based on the product of the reduction of perrhenate salts, such as the reduction of potassium perrhenate () by potassium metal. "Potassium rhenide" was shown to exist as a tetrahydrated complex, with the postulated chemical formula (potassium rhenide tetrahydrate). This compound exhibits strongly reducing properties, and slowly yields hydrogen gas when dissolved in water. The lithium and thallous salts were a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isostructural
Isostructural chemical compounds have similar chemical structures. " Isomorphous" when used in the relation to crystal structures is not synonymous: in addition to the same atomic connectivity that characterises isostructural compounds, isomorphous substances crystallise in the same space group and have the same unit cell dimensions. The IUCR definition used by crystallographers is: Examples include: *I- Gold(I) bromide is isostructural with gold(I) chloride *Borazine is isostructural with benzene * Indium(I) bromide is isostructural with β- thallium(I) iodide and has a distorted rock salt structure. Many minerals are isostructural when they differ only in the nature of a cation. Compounds which are isoelectronic usually have similar chemical structures. For example, methane, CH4, and the ammonium ion, NH4+, are isoelectric and are isostructural as both have a tetrahedral structure. The C-H and N-H bond lengths are different and crystal structures are completely different bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |