|
Isotopes Of Nickel
Naturally occurring nickel (Ni) consists of five stable isotopes; Ni, Ni, Ni, Ni and Ni; Ni is the most abundant (68.077% natural abundance). 26 radioisotopes have been characterized; the most stable are Ni with a half-life of 81,000 years, Ni with a half-life of 100.1 years, and Ni (6.077 days). All the other radioactive isotopes have half-lives of less than 60 hours and most of these have half-lives of less than 30 seconds. This element also has 8 meta states. List of isotopes , - , rowspan=3, , rowspan=3 style="text-align:right" , 28 , rowspan=3 style="text-align:right" , 20 , rowspan=3, 48.01952(46)# , rowspan=3, 2.8(8) ms , 2 p (70%) , , rowspan=3, 0+ , rowspan=3, , rowspan=3, , - , β+ (30%) , , - , β+, p? , , -id=Nickel-49 , rowspan=2, , rowspan=2 style="text-align:right" , 28 , rowspan=2 style="text-align:right" , 21 , rowspan=2, 49.00916(64)# , rowspan=2, 7.5(10) ms , β+, p (83%) , , rowspan=2, 7/2−# , rowspan=2, , rowspan=2, , - , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slow to react with air under standard conditions because a passivation layer of nickel oxide forms on the surface that prevents further corrosion. Even so, pure native nickel is found in Earth's crust only in tiny amounts, usually in ultramafic rocks, and in the interiors of larger nickel–iron meteorites that were not exposed to oxygen when outside Earth's atmosphere. Meteoric nickel is found in combination with iron, a reflection of the origin of those elements as major end products of supernova nucleosynthesis. An iron–nickel mixture is thought to compose Earth's outer and inner cores. Use of nickel (as natural meteoric nickel–iron alloy) has been traced as far back as 3500 BCE. Nickel was first isolated and classifie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an electron (the proton-to-electron mass ratio). Protons and neutrons, each with a mass of approximately one Dalton (unit), dalton, are jointly referred to as ''nucleons'' (particles present in atomic nuclei). One or more protons are present in the Atomic nucleus, nucleus of every atom. They provide the attractive electrostatic central force which binds the atomic electrons. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol ''Z''). Since each chemical element, element is identified by the number of protons in its nucleus, each element has its own atomic number, which determines the number of atomic electrons and consequently the chemical characteristi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive Decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered ''radioactive''. Three of the most common types of decay are Alpha decay, alpha, Beta decay, beta, and Gamma ray, gamma decay. The weak force is the Fundamental interactions, mechanism that is responsible for beta decay, while the other two are governed by the electromagnetic force, electromagnetic and nuclear forces. Radioactive decay is a randomness, random process at the level of single atoms. According to quantum mechanics, quantum theory, it is impossible to predict when a particular atom will decay, regardless of how long the atom has existed. However, for a significant number of identical atoms, the overall decay rate can be expressed as a decay constant or as a half-life. The half-lives of radioactive atoms have a huge range: f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Positron Emission
Positron emission, beta plus decay, or β+ decay is a subtype of radioactive decay called beta decay, in which a proton inside a radionuclide nucleus is converted into a neutron while releasing a positron and an electron neutrino (). Positron emission is mediated by the weak force. The positron is a type of beta particle (β+), the other beta particle being the electron (β−) emitted from the β− decay of a nucleus. An example of positron emission (β+ decay) is shown with magnesium-23 decaying into sodium-23: : → + + Because positron emission decreases proton number relative to neutron number, positron decay happens typically in large "proton-rich" radionuclides. Positron decay results in nuclear transmutation, changing an atom of one chemical element into an atom of an element with an atomic number that is less by one unit. Positron emission occurs extremely rarely in nature on Earth. Known instances include cosmic ray interactions and the decay of certain isotopes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron-56
Iron-56 (56Fe) is the most common isotope of iron. About 91.754% of all iron is iron-56. Of all nuclides, iron-56 has the lowest mass per nucleon. With 8.8 MeV binding energy per nucleon, iron-56 is one of the most tightly bound nuclei. The high nuclear binding energy for 56Fe represents the point where further nuclear reactions become energetically unfavorable. Because of this, it is among the heaviest elements formed in stellar nucleosynthesis reactions in massive stars. These reactions fuse lighter elements like magnesium, silicon, and sulfur to form heavier elements. Among the heavier elements formed is 56Ni, which subsequently decays to 56Co and then 56Fe. Relationship to nickel-62 Nickel-62, a relatively rare isotope of nickel, has a higher nuclear binding energy per nucleon; this is consistent with having a higher mass-per-nucleon because nickel-62 has a greater proportion of neutrons, which are slightly more massive than protons. (See the nickel-62 article ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Cobalt
Naturally occurring cobalt, Co, consists of a single stable isotope, Co (thus, cobalt is a mononuclidic element). Twenty-eight radioisotopes have been characterized; the most stable are Co with a half-life of 5.2714 years, Co (271.811 days), Co (77.236 days), and Co (70.844 days). All other isotopes have half-lives of less than 18 hours and most of these have half-lives of less than 1 second. This element also has 19 meta states, of which the most stable is 58m1Co with a half-life of 8.853 h. The isotopes of cobalt range in atomic weight from Co to Co. The main decay mode for isotopes with atomic mass less than that of the stable isotope, Co, is electron capture and the main mode of decay for those of greater than 59 atomic mass units is beta decay. The main decay products before Co are iron isotopes and the main products after are nickel isotopes. Radioisotopes can be produced by various nuclear reactions. For example, Co is produced by cyclotron irradiation of iron. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
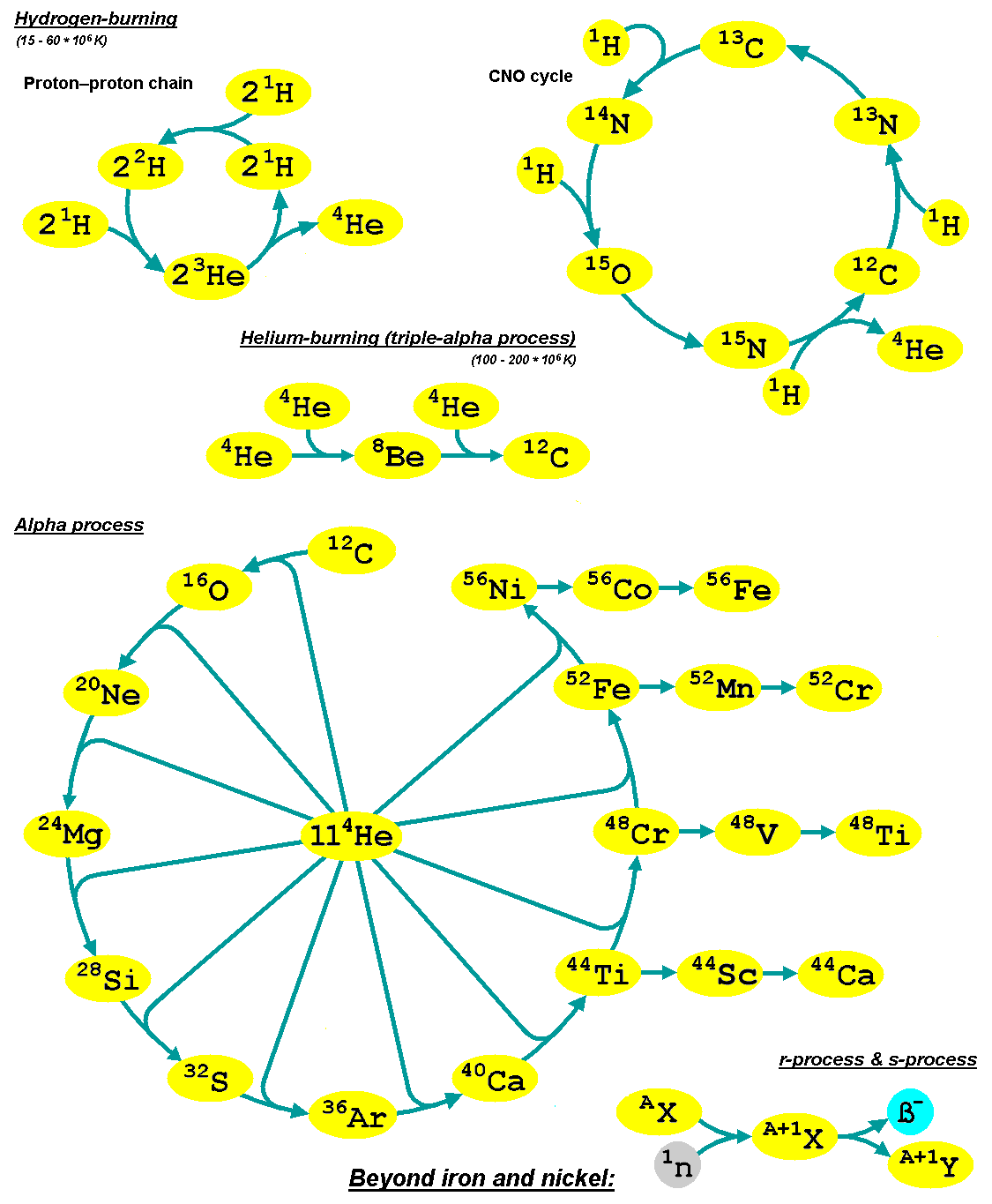
Alpha Process
The alpha process, also known as alpha capture or the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements. The other class is a cycle of reactions called the triple-alpha process, which consumes only helium, and produces carbon. The alpha process most commonly occurs in massive stars and during supernovae. Both processes are preceded by hydrogen fusion, which produces the helium that fuels both the triple-alpha process and the alpha ladder processes. After the triple-alpha process has produced enough carbon, the alpha-ladder begins and fusion reactions of increasingly heavy elements take place, in the order listed below. Each step only consumes the product of the previous reaction and helium. The later-stage reactions which are able to begin in any particular star, do so while the prior stage reactions are still under way in outer layers of the star. :\begin \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon-28
Silicon (14Si) has 25 known isotopes, with mass numbers ranging from 22 to 46. 28Si (the most abundant isotope, at 92.23%), 29Si (4.67%), and 30Si (3.1%) are stable. The longest-lived radioisotope is 32Si, which is produced by cosmic ray spallation of argon. Its half-life has been determined to be approximately 150 years (with decay energy 0.21 MeV), and it decays by beta emission to 32 P (which has a 14.27-day half-life) and then to 32 S. After 32Si, 31Si has the second longest half-life at 157.3 minutes. All others have half-lives under 7 seconds. List of isotopes , -id=Silicon-22 , rowspan=3, 22Si , rowspan=3 style="text-align:right" , 14 , rowspan=3 style="text-align:right" , 8 , rowspan=3, 22.03611(54)# , rowspan=3, 28.7(11) ms , β+, p (62%) , 21Mg , rowspan=3, 0+ , rowspan=3, , rowspan=3, , - , β+ (37%) , 22Al , - , β+, 2p (0.7%) , 20Na , -id=Silicon-23 , rowspan=3, 23Si , rowspan=3 style="text-align:right" , 14 , r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon-burning Process
In astrophysics, silicon burning is a very brief sequence of nuclear fusion reactions that occur in massive stars with a minimum of about 8–11 solar masses. Silicon burning is the final stage of fusion for massive stars that have run out of the fuels that power them for their long lives in the main sequence on the Hertzsprung–Russell diagram. It follows the previous stages of hydrogen, helium, carbon, neon and oxygen burning processes. Silicon burning begins when gravitational contraction raises the star's core temperature to 2.7–3.5 billion kelvin ( GK). The exact temperature depends on mass. When a star has completed the silicon-burning phase, no further fusion is possible. The star catastrophically collapses and may explode in what is known as a Type II supernova. Nuclear fusion sequence and silicon photodisintegration After a star completes the oxygen-burning process, its core is composed primarily of silicon and sulfur. If it has sufficiently high mass, it further ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fusion
Nuclear fusion is a nuclear reaction, reaction in which two or more atomic nuclei combine to form a larger nuclei, nuclei/neutrons, neutron by-products. The difference in mass between the reactants and products is manifested as either the release or absorption (electromagnetic radiation), absorption of energy. This difference in mass arises as a result of the difference in nuclear binding energy between the atomic nuclei before and after the fusion reaction. Nuclear fusion is the process that powers all active stars, via many Stellar nucleosynthesis, reaction pathways. Fusion processes require an extremely large Lawson criterion, triple product of temperature, density, and confinement time. These conditions occur only in Stellar core, stellar cores, advanced Nuclear weapon design, nuclear weapons, and are approached in List of fusion experiments, fusion power experiments. A nuclear fusion process that produces atomic nuclei lighter than nickel-62 is generally exothermic, due t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stellar Evolution
Stellar evolution is the process by which a star changes over the course of time. Depending on the mass of the star, its lifetime can range from a few million years for the most massive to trillions of years for the least massive, which is considerably longer than the current age of the universe. The table shows the lifetimes of stars as a function of their masses. All stars are formed from Gravitational collapse, collapsing clouds of gas and dust, often called nebulae or molecular clouds. Over the course of millions of years, these protostars settle down into a state of equilibrium, becoming what is known as a main sequence star. Nuclear fusion powers a star for most of its existence. Initially the energy is generated by the fusion of hydrogen atoms at the stellar core, core of the main-sequence star. Later, as the preponderance of atoms at the core becomes helium, stars like the Sun begin to fuse hydrogen along a spherical shell surrounding the core. This process causes the st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supernova
A supernova (: supernovae or supernovas) is a powerful and luminous explosion of a star. A supernova occurs during the last stellar evolution, evolutionary stages of a massive star, or when a white dwarf is triggered into runaway nuclear fusion. The original object, called the ''progenitor'', either collapses to a neutron star or black hole, or is completely destroyed to form a diffuse nebula. The peak optical luminosity of a supernova can be comparable to that of an entire galaxy before fading over several weeks or months. The last supernova directly observed in the Milky Way was Kepler's Supernova in 1604, appearing not long after Tycho's Supernova in 1572, both of which were visible to the naked eye. The supernova remnant, remnants of more recent supernovae have been found, and observations of supernovae in other galaxies suggest they occur in the Milky Way on average about three times every century. A supernova in the Milky Way would almost certainly be observable through mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |