|
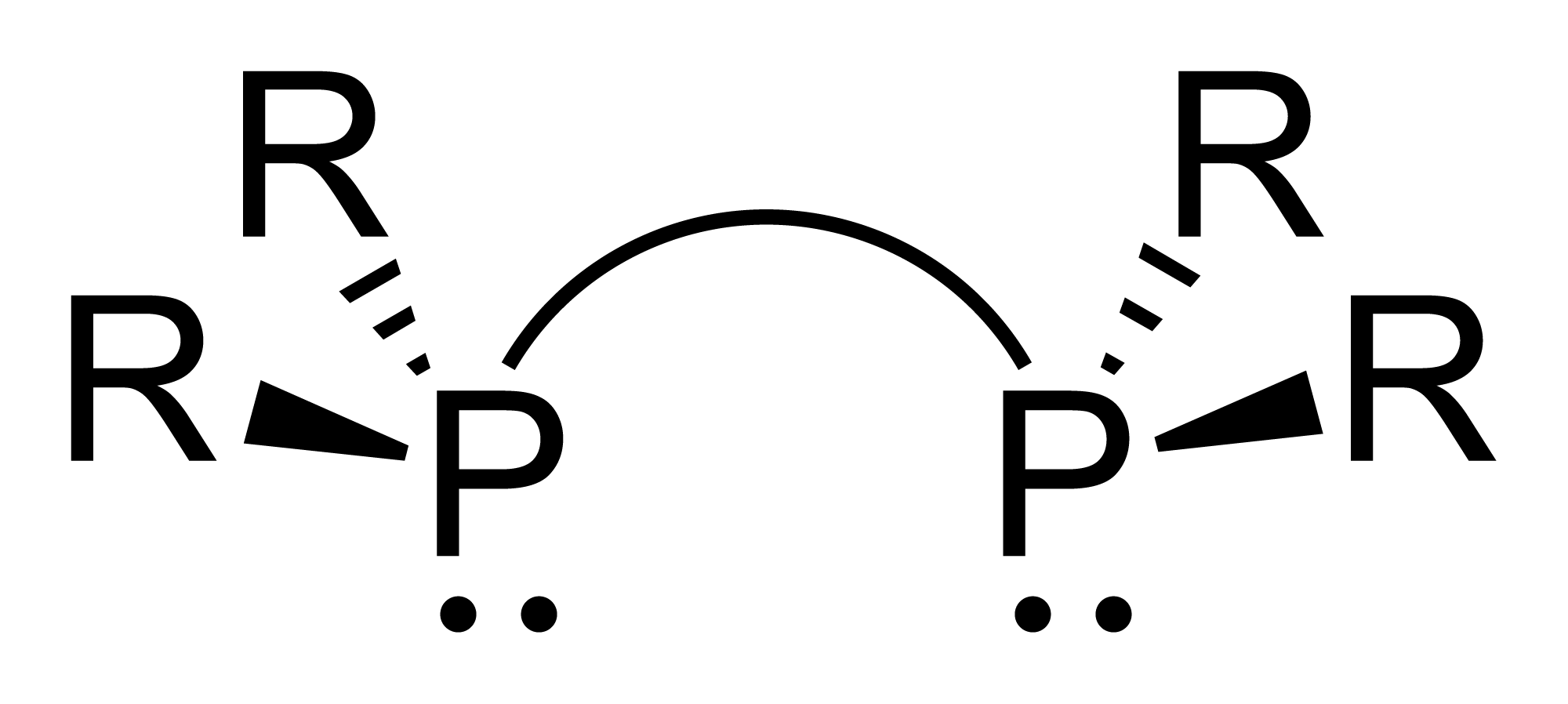
Diphosphines
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligand, phosphine ligands in inorganic chemistry, inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelate, chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in Homogeneous catalysis, homogeneous catalysts. Synthesis image:IPr2PCl.png, 220px, Chlorodiisopropylphosphine is a popular building block for the preparation of diphosphines. From phosphide building blocks Many widely used diphosphine ligands have the general formula Ar2P(CH2)nPAr2. These compounds can be prepared from the reaction of X(CH2)nX (X=halogen) and MPPh2 (M = alkali metal): :Cl(CH2)nCl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dcpe
Bis(dicyclohexylphosphino)ethane, abbreviated dcpe, is an organophosphorus compound with the formula (C6H11)2PCH2CH2P(C6H11)2. It is a white solid that is soluble in nonpolar organic solvents. The compound is used as a bulky and highly basic diphosphine ligand in coordination chemistry.Sowa, J. R., Jr., Zanotti, V., Facchin, G., Angelici, R. J., "Calorimetric studies of the heats of protonation of the metal in Fe(CO)3(bidentate phosphine, arsine) complexes: effects of chelate ligands on metal basicity", J. Am. Chem. Soc. 1992, 114, 160. References {{DEFAULTSORT:Bis(dicyclohexylphosphino)ethane, 1,2- Chelating agents Diphosphines 1,2-Ethanediyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1-Bis(diphenylphosphino)methane
1,1-Bis(diphenylphosphino)methane (dppm), is an organophosphorus compound with the formula CH2(PPh2)2. Dppm, a white, crystalline powder, is used in inorganic and organometallic chemistry as a ligand. It is more specifically a chelating ligand because it is a ligand that can bond to metals with two phosphorus donor atoms. The natural bite angle is 73°. Synthesis and reactivity 1,1-Bis(diphenylphosphino)methane was first prepared by the reaction of sodium diphenylphosphide (Ph2PNa) with dichloromethane: :Ph3P + 2 Na → Ph2PNa + NaPh :2NaPPh2 + CH2Cl2 → Ph2PCH2PPh2 + 2 NaCl The methylene group (CH2) in dppm (and especially its complexes) is mildly acidic. The ligand can be oxidized to give the corresponding oxides and sulfides CH2 (E)Ph2sub>2 (E = O, S). The methylene group is even more acidic in these derivatives. Coordination chemistry As a chelating ligand, 1,1-bis(diphenylphosphino)methane forms a four-membered ring with the constituents MP2C. The ligand promotes the forma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphine Ligand
A metal-phosphine complex is a coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0). Preparation Many metal phosphine complexes are prepared by reactions of metal halides with preformed phosphines. For example, treatment of a suspension of palladium chloride in ethanol with triphenylphosphine yields monomeric bis(triphenylphosphine)palladium(II) chloride units. : dCl2sub>n + 2PPh3 → PdCl2(PPh3)2 The first reported phosphine complexes were ''cis''- and ''trans''-PtCl2(PEt3)2 reported by Cahours and Gal in 1870. Often the phosphine serves both as a ligand and as a reductant. This property is illustrated by the synthesis of many platinum-metal complexes of triphenylp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bis(dicyclohexylphosphino)ethane
Bis(dicyclohexylphosphino)ethane, abbreviated dcpe, is an organophosphorus compound with the formula (C6H11)2PCH2CH2P(C6H11)2. It is a white solid that is soluble in nonpolar organic solvents. The compound is used as a bulky and highly basic diphosphine ligand in coordination chemistry A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ....Sowa, J. R., Jr., Zanotti, V., Facchin, G., Angelici, R. J., "Calorimetric studies of the heats of protonation of the metal in Fe(CO)3(bidentate phosphine, arsine) complexes: effects of chelate ligands on metal basicity", J. Am. Chem. Soc. 1992, 114, 160. References {{DEFAULTSORT:Bis(dicyclohexylphosphino)ethane, 1,2- Chelating agents Diphosphines 1,2-Ethanediyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DIPAMP
DIPAMP is an organophosphorus compound that is used as a ligand in homogeneous catalysis. It is a white solid that dissolves in organic solvents. Work on this compound by W. S. Knowles was recognized with the Nobel Prize in Chemistry. DIPAMP was the basis for one of the first industrial scale asymmetric hydrogenation, the synthesis of the drug L-DOPA. : DIPAMP is a C2-symmetric diphosphine. Each phosphorus Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ... centre, which is pyramidal, bears three different substituents - anisyl, phenyl, and the ethylene group. The ligand therefore exists as the enantiomeric (''R'',''R'') and (''S'',''S'') pair, as well as the achiral ''meso'' isomer. DIPAMP was originally prepared by an oxidative coupling, starting from anisyl(phenyl)(methyl)p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dppbz
1,2-Bis(diphenylphosphino)benzene (dppbz) is an organophosphorus compound with the formula C6H4(PPh2)2 ( Ph = C6H5). Classified as a diphosphine ligand, it is a common bidentate ligand in coordination chemistry A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' .... It is a white, air-stable solid. As a chelating ligand, dppbz is very similar to 1,2-bis(diphenylphosphino)ethylene. References {{DEFAULTSORT:Bis(diphenylphosphino)benzene, 1,2- Chelating agents Diphosphines Phenyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Bis(diisopropylphosphino)ethane
1,2-Bis(diisopropylphosphino)ethane (dippe) is a commonly used bidentate ligand In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ... in coordination chemistry. This compound is similar to the ligand 1,2-bis(diphenylphosphino)ethane (dppe), with the substitution of isopropyl groups for phenyl groups. {{DEFAULTSORT:Bis(diisopropylphosphino)ethane, 1,2- Diphosphines Isopropyl compounds 1,2-Ethanediyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Bis(dimethylphosphino)ethane
1,2-Bis(dimethylphosphino)ethane (dmpe) is a diphosphine ligand in coordination chemistry. It is a colorless, air-sensitive liquid that is soluble in organic solvents. With the formula (CHPMe), dmpe is used as a compact strongly basic spectator ligand (Me = methyl In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as ...), Representative complexes include V(dmpe)(BH), Mn(dmpe)(AlH), Tc(dmpe)(CO)Cl, and Ni(dmpe)Cl. Synthesis It is synthesised by the reaction of methylmagnesium iodide with 1,2-bis(dichlorophosphino)ethane: :ClPCHCHPCl + 4 MeMgI → MePCHCHPMe + 4 MgICl Alternatively it can be generated by alkylation of sodium dimethylphosphide. The synthesis of dmpe from thiophosphoryl chloride has led to serious accidents and has been abandoned. Related ligands * Bis(dicyclohexy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Bis(diphenylphosphino)ethane
1,2-Bis(diphenylphosphino)ethane (dppe) is an organophosphorus compound with the formula (PhPCH) (Ph = phenyl). It is a common symmetrical bidentate ligand in coordination chemistry. It is a white solid that is soluble in organic solvents. Preparation The preparation of dppe entails the alkylation of NaP(C6H5)2 with 1,2-dichloroethane: : Reactions The reduction of dppe by lithium give the disecondary phosphine: : Hydrolysis gives the bis(secondary phosphine). : : Treatment of dppe with hydrogen peroxide produces the phosphine oxides .Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley & Sons, Ltd Selective mono-oxidation of dppe can be achieved by benzylation followed by hydrolysis: : : Hydrogenation of dppe gives the ligand bis(dicyclohexylphosphino)ethane. Coordination complexes Many coordination complexes of dppe are known, and some are used as homogeneous catalysts. Dppe is almost invariably chelating, although there are examples of monodentate (e.g., W(CO)(d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DPPE Structure
DPPE may refer to the chemicals: * 1,2-Bis(diphenylphosphino)ethane 1,2-Bis(diphenylphosphino)ethane (dppe) is an organophosphorus compound with the formula (PhPCH) (Ph = phenyl). It is a common symmetrical bidentate ligand in coordination chemistry. It is a white solid that is soluble in organic solvents. Prepa ... * Dipalmitoylphosphatidylethanolamine {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Bis(diisopropylphosphino)ethane-2D-by-AHRLS-2012
Onekama ( ) is a village in Manistee County in the U.S. state of Michigan. The population was 399 at the 2020 census. The village is located on the northeast shore of Portage Lake and is surrounded by Onekama Township. The town's name is derived from ''Ona-ga-maa'', an Anishinaabe word which means "singing water". History The predecessor of the village of Onekama was the settlement of Portage at Portage Point, first established in 1845, at the western end of Portage Lake, at the outlet of Portage Creek. In 1871, when landowners around the land-locked lake became exasperated with the practices of the Portage Sawmill, they took the solution into their own hands and dug a channel through the narrow isthmus, opening a waterway that lowered the lake by and brought it to the same level as Lake Michigan. When this action dried out Portage Creek on May 14, 1871, the settlement, which had only the week before been designated as "Onekama" with a post office under that name, moved to t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |