|
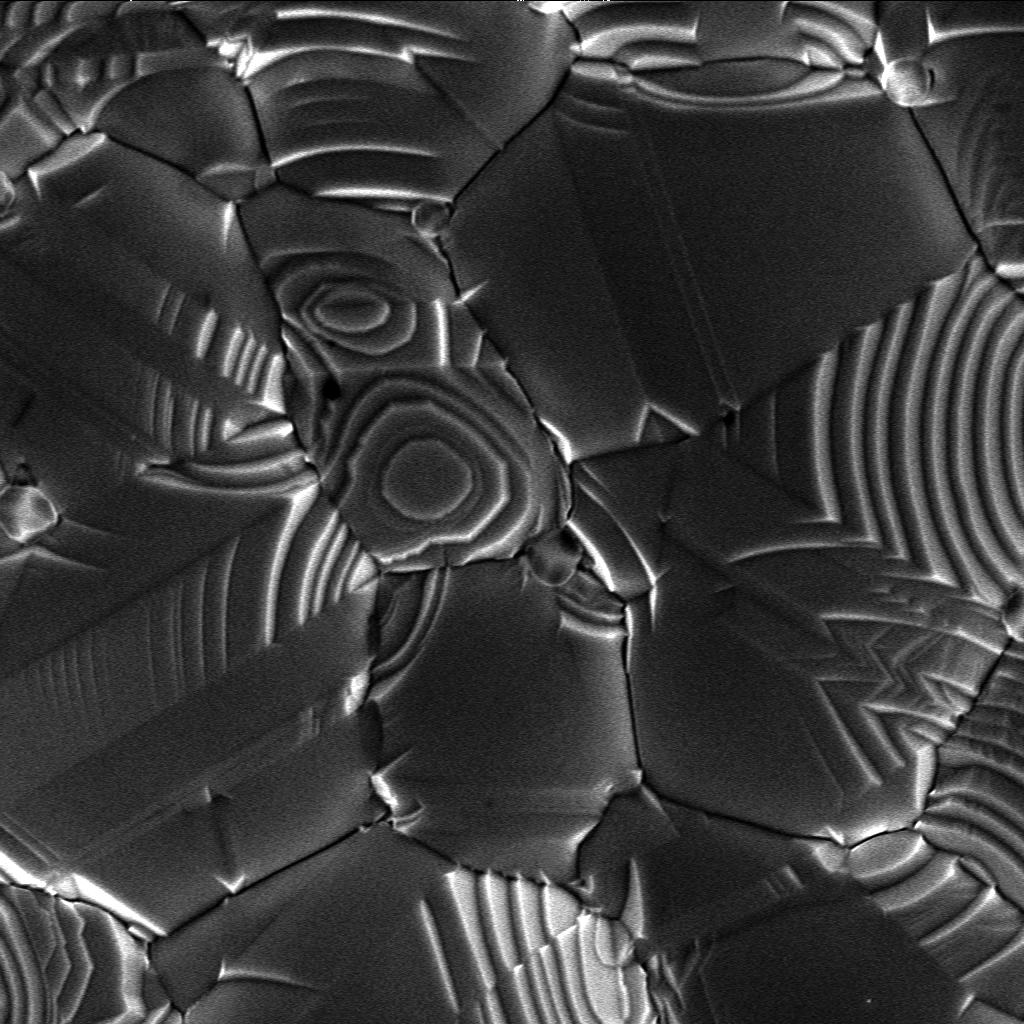
Crystal Growth
Crystal growth is a major stage of a crystallization, crystallization process, and consists of the addition of new atoms, ions, or polymer strings into the characteristic arrangement of the crystalline lattice. The growth typically follows an initial stage of either homogeneous or heterogeneous (surface catalyzed) nucleation, unless a "seed" crystal, purposely added to start the growth, was already present. The action of crystal growth yields a crystalline solid whose atoms or molecules are close packed, with fixed positions in space relative to each other. The crystalline states of matter, state of matter is characterized by a distinct structural rigidity and very high resistance to Plastic deformation in solids, deformation (i.e. changes of shape and/or volume). Most crystalline solids have high values both of Young's modulus and of the shear modulus of elasticity (physics), elasticity. This contrasts with most liquids or fluids, which have a low shear modulus, and typically exh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schematic Of Crystal Growth Of Simple Cubic Lattice, Showing Additional Molecule Adding In Corner
A schematic, or schematic diagram, is a designed representation of the elements of a system using abstract, graphic symbols rather than realistic pictures. A schematic usually omits all details that are not relevant to the key information the schematic is intended to convey, and may include oversimplified elements in order to make this essential meaning easier to grasp, as well as additional organization of the information. For example, a subway map intended for passengers may represent a subway station with a dot. The dot is not intended to resemble the actual station at all but aims to give the viewer information without unnecessary visual clutter. A schematic diagram of a chemical process uses symbols in place of detailed representations of the vessels, piping, valves, pumps, and other equipment that compose the system, thus emphasizing the functions of the individual elements and the interconnections among them and suppresses their physical details. In an electronic circuit d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homogeneous
Homogeneity and heterogeneity are concepts relating to the uniformity of a substance, process or image. A homogeneous feature is uniform in composition or character (i.e., color, shape, size, weight, height, distribution, texture, language, income, disease, temperature, radioactivity, architectural design, etc.); one that is heterogeneous is distinctly nonuniform in at least one of these qualities. Etymology and spelling The words ''homogeneous'' and ''heterogeneous'' come from Medieval Latin ''homogeneus'' and ''heterogeneus'', from Ancient Greek ὁμογενής (''homogenēs'') and ἑτερογενής (''heterogenēs''), from ὁμός (''homos'', "same") and ἕτερος (''heteros'', "other, another, different") respectively, followed by γένος (''genos'', "kind"); -ous is an adjectival suffix. Alternate spellings omitting the last ''-e-'' (and the associated pronunciations) are common, but mistaken: ''homogenous'' is strictly a biological/pathological term whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
On The Equilibrium Of Heterogeneous Substances
In the history of thermodynamics, "On the Equilibrium of Heterogeneous Substances" is a 300-page paper written by American chemical physicist Willard Gibbs. It is one of the founding papers in thermodynamics, along with German physicist Hermann von Helmholtz's 1882 paper "'' Thermodynamik chemischer Vorgänge''." Together they form the foundation of chemical thermodynamics as well as a large part of physical chemistry. Gibbs's paper marked the beginning of chemical thermodynamics by integrating chemical, physical, electrical, and electromagnetic phenomena into a coherent system. It introduced concepts such as chemical potential, phase rule, and more, which form the basis for modern physical chemistry. American writer Bill Bryson describes Gibbs's paper as "the '' Principia'' of thermodynamics". "On the Equilibrium of Heterogeneous Substances", was originally published in a relatively obscure American journal, the '' Transactions of the Connecticut Academy of Arts and Sciences ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Josiah Willard Gibbs
Josiah Willard Gibbs (; February 11, 1839 – April 28, 1903) was an American mechanical engineer and scientist who made fundamental theoretical contributions to physics, chemistry, and mathematics. His work on the applications of thermodynamics was instrumental in transforming physical chemistry into a rigorous deductive science. Together with James Clerk Maxwell and Ludwig Boltzmann, he created statistical mechanics (a term that he coined), explaining the laws of thermodynamics as consequences of the statistical properties of ensembles of the possible states of a physical system composed of many particles. Gibbs also worked on the application of Maxwell's equations to problems in physical optics. As a mathematician, he created modern vector calculus (independently of the British scientist Oliver Heaviside, who carried out similar work during the same period) and described the Gibbs phenomenon in the theory of Fourier analysis. In 1863, Yale University awarded Gibbs the firs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Morphology (chemistry)
Morphology, from the Greek and meaning "study of shape", may refer to: Disciplines *Morphology (archaeology), study of the shapes or forms of artifacts *Morphology (astronomy), study of the shape of astronomical objects such as nebulae, galaxies, or other extended objects *Morphology (biology), the study of the form or shape of an organism or part thereof *Morphology (folkloristics), the structure of narratives such as folk tales *Morphology (linguistics), the study of the structure and content of word forms *Morphology (sociology), the analysis of the typical social form taken by human relations and practices *Mathematical morphology, a theoretical model based on lattice theory, used for digital image processing * River morphology, the field of science dealing with changes of river platform * Urban morphology, study of the form, structure, formation and transformation of human settlements *Geomorphology, the study of landforms *Morphology (architecture and engineering), research wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid below its freezing point without it becoming a solid. Per the established international definition, supercooling means ''‘cooling a substance below the normal freezing point without solidification’.'' IIR International Dictionary of Refrigeration, http://dictionary.iifiir.org/search.php ASHRAE Terminology, https://www.ashrae.org/technical-resources/free-resources/ashrae-terminology While it can be achieved by different physical means, the postponed solidification is most often due to the absence of nucleation, seed crystals or nuclei around which a crystal structure can form. The supercooling of water can be achieved without any special techniques other than chemical demineralization, down to . Supercooled water can occur naturally, for example in the atmosphere, animals or plants. This phenomenon was first identified in 1724 by Daniel Gabriel Fahrenheit, while developing Fahren ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solidification
Freezing is a phase transition in which a liquid turns into a solid when its temperature is lowered below its freezing point. For most substances, the melting and freezing points are the same temperature; however, certain substances possess differing solid-liquid transition temperatures. For example, agar displays a Hysteresis#Liquid–solid-phase transitions, hysteresis in its melting point and freezing point. It melts at and solidifies from . Crystallization Most liquids freeze by crystallization, formation of crystal, crystalline solid from the uniform liquid. This is a first-order thermodynamic phase transition, which means that as long as solid and liquid coexist, the temperature of the whole system remains very nearly equal to the melting point due to the slow removal of heat when in contact with air, which is a poor heat conductor. Because of the latent heat of fusion, the freezing is greatly slowed and the temperature will not drop anymore once the freezing starts but ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Philosophical Transactions Of The Royal Society A
''Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences'' is a fortnightly peer-reviewed scientific journal published by the Royal Society. It publishes original research and review content in a wide range of physical scientific disciplines. Articles can be accessed online a few months prior to the printed journal. All articles become freely accessible two years after their publication date. The current editor-in-chief is John Dainton. Overview ''Philosophical Transactions of the Royal Society A'' publishes themed journal issues on topics of current scientific importance and general interest within the physical, mathematical and engineering sciences, edited by leading authorities and comprising original research, reviews and opinions from prominent researchers. Past issue titles include "Supercritical fluids - green solvents for green chemistry?", "Tsunamis: Bridging science, engineering and society", "Spatial transformations ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicolás Cabrera (physicist)
Nicolás Cabrera (1913–1989), was a Spanish physicist who did important work on the theories of crystal growth and the oxidisation of metals. He is known for the development of the Burton–Cabrera–Frank theory for crystal growth, with Keith Burton and Charles Frank. He was the son of another famous Spanish physicist Blas Cabrera and the father of American Physicist Blas Cabrera Navarro. He spent many years in exile during the Francoist State. He was Professor of the Department of physics in the University of Virginia, where he worked from 1952. He became known for his interests in engineering and material science. He founded the physics department and was a professor at the Autonomous University of Madrid (UAM), from 1971. He is considered to have given an impulse to the study of physics in Spain from the time of his return. For a time Javier Solana Francisco Javier Solana de Madariaga CYC (; born 14 July 1942) is a Spanish physicist and PSOE politician. After ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Keith Burton
William Keith Burton (known as Keith Burton) FRSE (12 October 1922 – 30 December 1996) was an English electrical engineer and theoretical physicist. Life He was born in Manchester and attended Manchester Grammar School prior to studying Electrical Engineering at Manchester College of Technology. After graduating he gained a job with GEC, first at Heywood, then Wembley. From 1945 until 1951 he was employed as a theoretical physicist with ICI Ltd, being seconded to Bristol University for the final 4 years, lecturing under Nevill Francis Mott and Herbert Fröhlich. From 1951 onwards he lectured in Natural Philosophy at Glasgow University. He developed the Burton–Carbrera–Frank model for adatoms and crystal growth, with Nicolás Cabrera and Frederick Charles Frank. He was elected a Fellow of the Royal Society of Edinburgh on 3 March 1958. He died at his home in Milngavie near Glasgow Glasgow is the Cities of Scotland, most populous city in Scotland, located on th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |