|
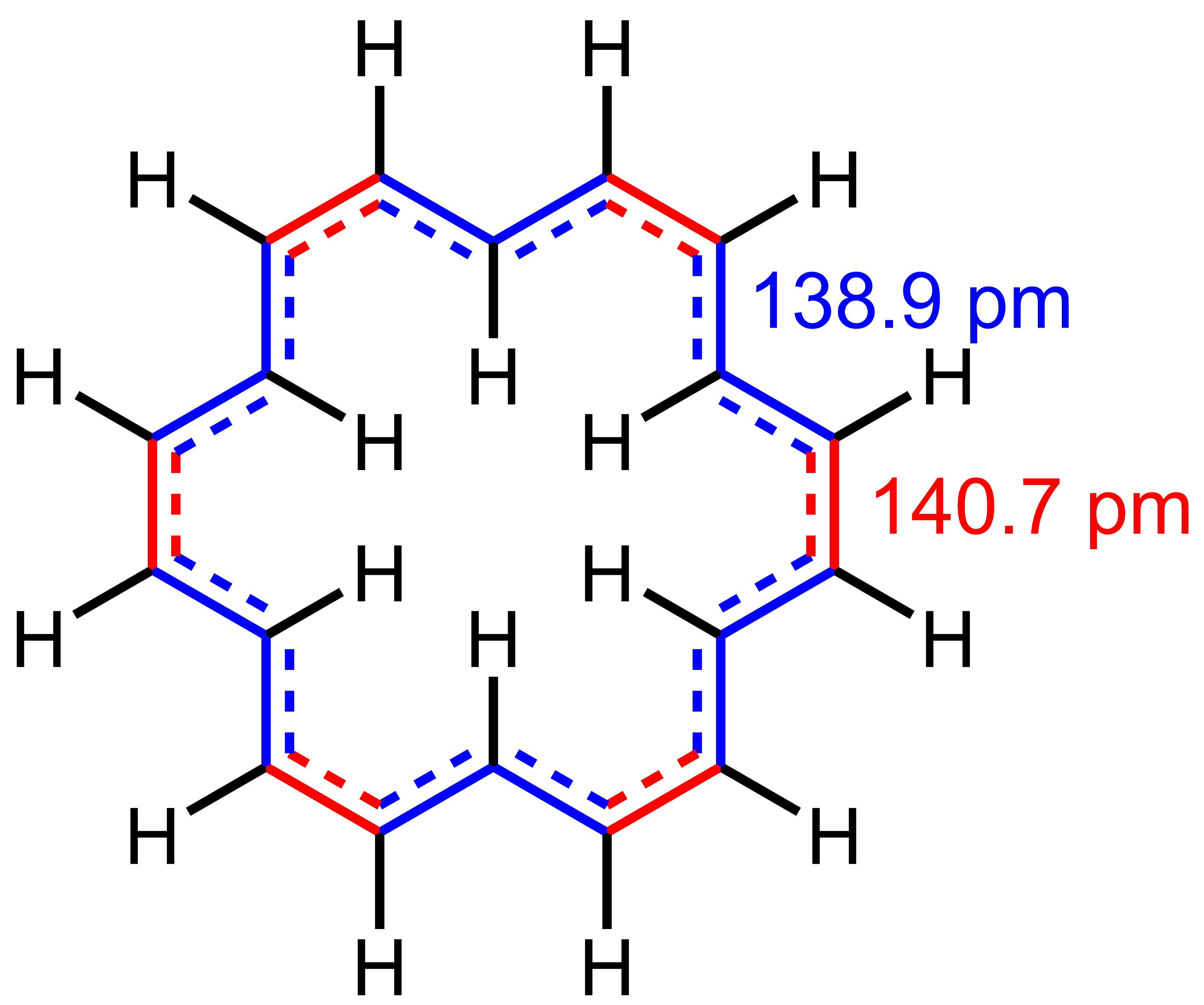
Coronene
Coronene (also known as superbenzene and cyclobenzene) is a polycyclic aromatic hydrocarbon (PAH) comprising seven peri-fused benzene rings. Its chemical formula is . It is a yellow material that dissolves in common solvents including benzene, toluene, and dichloromethane. Its solutions emit blue light fluorescence under UV light. It has been used as a solvent probe, similar to pyrene. The compound is of theoretical interest to organic chemists because of its aromaticity. It can be described by 20 resonance structures or by a set of three mobile Clar sextets. In the Clar sextet case, most stable structure for coronene has only three isolated outer sextets as fully aromatic although superaromaticity would still be possible when these sextets are able to migrate into next ring. Occurrence and synthesis Coronene occurs naturally as the very rare mineral carpathite, characterized by flakes of pure coronene embedded in sedimentary rock. This mineral may be created from ancient ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hexa-peri-hexabenzocoronene
Hexa-peri-hexabenzocoronene (HBC) is a polycyclic aromatic hydrocarbon with the molecular formula C42H18. It consists of a central coronene molecule, with an additional benzene ring fused between each adjacent pair of rings around the periphery. It is sometimes simply called hexabenzocoronene, however, there are other chemicals that share this less-specific name, such as hexa-cata-hexabenzocoronene. Hexa-peri-hexabenzocoronene has been imaged by atomic force microscopy (AFM) providing the first example of a molecule in which differences in bond order and bond lengths of the individual bonds can be distinguished by a measurement in Position and momentum space, direct space. Supramolecular structures Various hexabenzocoronenes have been investigated in supramolecular electronics. They are known to Molecular self-assembly, self-assemble into a columnar phase. One derivative in particular forms carbon nanotubes with interesting electrical properties. The columnar phase in this comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hexa-cata-hexabenzocoronene
Hexa-''cata''-hexabenzocoronene (hexabenzo 'a'',''d'',''g'',''j'',''m'',''p''oronene) is a polycyclic aromatic hydrocarbon with the molecular formula C48H24. It consists of a central coronene molecule, with an additional benzene ring fused onto each ring around the periphery. Hexa-''cata''-hexabenzocoronene has a contorted structure due to steric crowding among the benzene rings around the edge, analogous to the situation in benzo 'c''henanthrene. See also * Hexabenzocoronene Hexa-peri-hexabenzocoronene (HBC) is a polycyclic aromatic hydrocarbon with the molecular formula C42H18. It consists of a central coronene molecule, with an additional benzene ring fused between each adjacent pair of rings around the periphery. ... References External links * Polycyclic aromatic hydrocarbons {{hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Polycyclic Aromatic Hydrocarbon
A Polycyclic aromatic hydrocarbon (PAH) is any member of a class of organic compounds that is composed of multiple fused aromatic rings. Most are produced by the incomplete combustion of organic matter— by engine exhaust fumes, tobacco, incinerators, in roasted meats and cereals, or when biomass burns at lower temperatures as in forest fires. The simplest representative is naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are also found in fossil fuel deposits such as coal and in petroleum. Exposure to PAHs can lead to different types of cancer, to fetal development complications, and to cardiovascular issues. Polycyclic aromatic hydrocarbons are discussed as possible starting materials for abiotic syntheses of materials required by the earliest forms of life. Nomenclature and structure The terms polyaromatic hydrocarbon, or polynuclear aromatic hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Coronene MOF
Coronene (also known as superbenzene and cyclobenzene) is a polycyclic aromatic hydrocarbon (PAH) comprising seven peri-fused benzene rings. Its chemical formula is . It is a yellow material that dissolves in common solvents including benzene, toluene, and dichloromethane. Its solutions emit blue light fluorescence under UV light. It has been used as a solvent probe, similar to pyrene. The compound is of theoretical interest to organic chemists because of its aromaticity. It can be described by 20 resonance structures or by a set of three mobile Clar's rule, Clar sextets. In the Clar sextet case, most stable structure for coronene has only three isolated outer sextets as fully aromatic although superaromaticity would still be possible when these sextets are able to migrate into next ring. Occurrence and synthesis Coronene occurs naturally as the very rare mineral carpathite, characterized by flakes of pure coronene embedded in sedimentary rock. This mineral may be created from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Dicoronylene
Dicoronylene is the trivial name for a very large polycyclic aromatic hydrocarbon. It has 15 rings and is a brick-red solid. Its formula is . Dicoronylene sublimes under high vacuum, 0.001 torr, between 250 °C and 300 °C. Structure Due to its large size and limited availability, the organic chemistry of dicoronylene is little known. Dicoronylene does undergo a Diels–Alder reaction with maleic anhydride on one or both of the central bay regions on either side of the bridging ring. The double bond of maleic anhydride forms two carbon–carbon bonds on the ends of the bay region, making a new six-membered ring. Heating removes the anhydride as carbon dioxide gas and gives the corresponding 16-ring and 17-ring PAHs. Occurrence Dicoronylene was first observed in the solid residue produced in coal gasification. This residue contained large amounts of coronene and ovalene. After these were extracted and identified, a reddish residue remained, which was sparingly soluble in organic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carpathite
Carpathite is a very rare hydrocarbon mineral, consisting of exceptionally pure coronene (C24H12), a polycyclic aromatic hydrocarbon. The name has been spelled karpatite and the mineral was improperly renamed pendletonite. Discovery The mineral was first described in 1955 for an occurrence in Transcarpathian Oblast, Ukraine. It was named for the Carpathian Mountains. In 1967, unaware of the earlier description, Joseph Murdoch analyzed and described a specimen from the Picacho Peak area of San Benito County, California and named it "pendletonite". Structure Carpathite has the same crystal structure of pure coronene. The molecules are planar and lie in two sets with roughly perpendicular orientations. Molecules in the same set are parallel and partially offset, with planes 0.3463 nm apart. That is slightly larger than the inter-layer distance of graphite layers (0.335 nm), and much larger than the C-C bond lengths within the molecule (about 0.14 nm). This "corrugate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Circulene
A circulene is a macrocyclic arene in which a central polygon is surrounded and fused by benzenoids. Nomenclature within this class of molecules is based on the number of benzene rings surrounding the core, which is equivalent to the size of the central polygon. Examples which have been synthesized include irculene ( corannulene), irculene ( coronene), irculene, and 2irculene ( kekulene) These compounds belong to a larger class of geodesic polyarenes. Whereas irculene is bowl-shaped and irculene is planar, irculene has a unique saddle-shaped structure (compare to cones and partial cones in calixarenes). The helicenes are a conceptually related class of structures in which the array of benzene rings form an open helix rather than a closed ring. Quadrannulene ( irculene) The simple irculene compound itself has not been synthesized, but a derivative, tetrabenzo irculene, also called quadrannulene, has. irculenes The isolation of the irculene derivative 2,5,6,9,10,13, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 49,000 in the world. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, People' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
CRC Handbook Of Chemistry And Physics
The ''CRC Handbook of Chemistry and Physics'' is a comprehensive one-volume reference resource for science research. First published in 1914, it is currently () in its 105th edition, published in 2024. It is known colloquially among chemists as the "Rubber Bible", as CRC originally stood for "Chemical Rubber Company". As late as the 1962–1963 edition (3604 pages), the ''Handbook'' contained myriad information for every branch of science and engineering. Sections in that edition include: Mathematics, Properties and Physical Constants, Chemical Tables, Properties of Matter, Heat, Hygrometric and Barometric Tables, Sound, Quantities and Units, and Miscellaneous. ''Mathematical Tables from Handbook of Chemistry and Physics'' was originally published as a supplement to the handbook up to the 9th edition (1952); afterwards, the 10th edition (1956) was published separately as '' CRC Standard Mathematical Tables''. Earlier editions included sections such as "Antidotes of Poisons", ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Cyclooctadecanonaene
Cyclooctadecanonaene or 8nnulene is an organic compound with chemical formula . It belongs to the class of highly conjugated compounds known as annulenes and is aromatic. The usual isomer that 8nnulene refers to is the most stable one, containing six interior hydrogens and twelve exterior ones, with the nine formal double bonds in the ''cis'',''trans'',''trans'',''cis'',''trans'',''trans'',''cis'',''trans'',''trans'' configuration. It is reported to be a red-brown crystalline solid. Aromaticity Notably, 8nnulene is the first annulene after benzene ( nnulene) to be fully aromatic: its π-system contains 4''n'' + 2 electrons (''n'' = 4), and it is large enough to comfortably accommodate six hydrogen atoms in its interior, allowing it to adopt a planar shape, thus satisfying Hückel's rule. The discovery of aromatic stabilization for 8nnulene is historically significant for confirming earlier theoretical predictions based on molecular orbital theory, since simple versions of vale ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating the presence of double bonds within the carbon structure. Graphene is known for its exceptionally high Ultimate tensile strength, tensile strength, Electrical resistivity and conductivity, electrical conductivity, Transparency and translucency, transparency, and being the thinnest two-dimensional material in the world. Despite the nearly transparent nature of a single graphene sheet, graphite (formed from stacked layers of graphene) appears black because it absorbs all visible light wavelengths. On a microscopic scale, graphene is the strongest material ever measured. The existence of graphene was first theorized in 1947 by P. R. Wallace, Philip R. Wallace during his research on graphite's electronic properties, while the term ''graphen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |