|
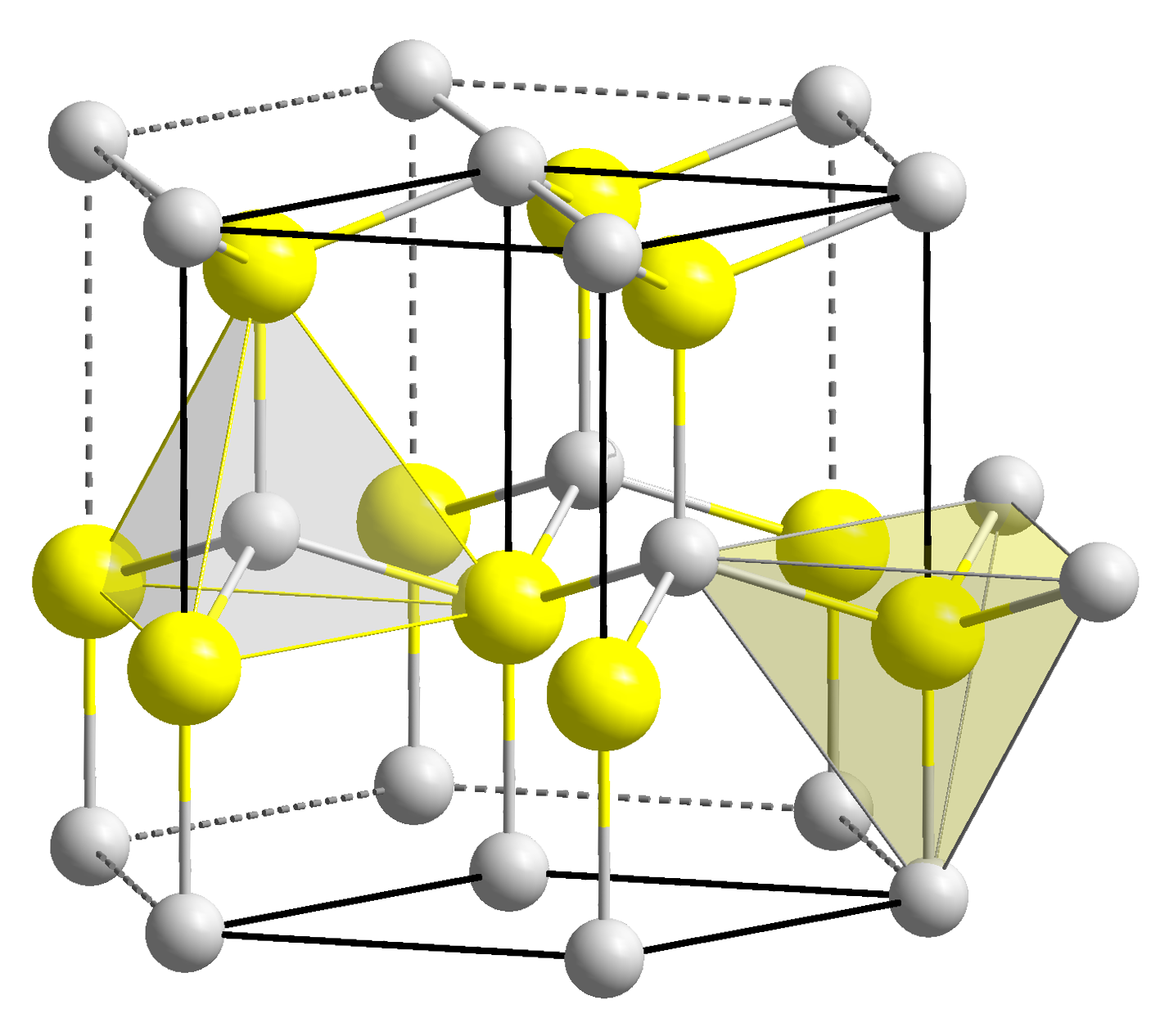
Copper Hydride
Copper hydride is an inorganic compound with the chemical formula CuHn where n ~ 0.95. It is a red solid, rarely isolated as a pure composition, that decomposes to the elements. Copper hydride is mainly produced as a reducing agent in organic synthesis and as a precursor to various catalysts. History In 1844, the French chemist Adolphe Wurtz synthesised copper hydride for the first time. He reduced an aqueous solution of copper(II) sulfate with hypophosphorous acid (H3PO2). In 2011, Panitat Hasin and Yiying Wu were the first to synthesise a metal hydride (copper hydride) using the technique of sonication. Copper hydride has the distinction of being the first metal hydride discovered. In 2013, it was established by Donnerer et al. that, at least up to fifty gigapascals, copper hydride cannot be synthesised by pressure alone. However, they were successful in synthesising several copper-hydrogen alloys under pressure. Chemical properties Structure In copper hydride, elem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep Mantle (geology), mantle remain active areas of investigation. All allotropes (structurally different pure forms of an element) and some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, graphene, etc.), carbon monoxide , carbon dioxide , carbides, and salt (chemistry), salts of inorganic anions such as carbonates, cyanides, cyanates, thiocyanates, isothiocyanates, etc. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it cannot occur within life, living things. History ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stryker's Reagent
Stryker's reagent ( PPh3)CuHsub>6), also known as the Osborn complex, is a hexameric copper hydride ligated with triphenylphosphine. It is a brick red, air-sensitive solid. Stryker's reagent is a mildly hydridic reagent, used in homogeneous catalysis of conjugate reduction reactions of enones, enoates, and related substrates. Preparation and structure The compound is prepared by adding sodium trimethoxyborohydride to a solution of Ph3CuClsub>4 in DMF, after which it precipitates out as a DMF complex ( Cu(PPh3)sub>6•DMF). Other more convenient methods have been developed since its discovery. In terms of its structure, the compound is an octahedral cluster of Cu(PPh3) centres that are bonded by Cu---Cu and Cu---H interactions. Originally six of the eight faces were thought to be capped by hydride ligands. This structural assignment was revised in 2014; the hydrides are now best described as edge bridging rather than face bridging. Applications in organic synthesis The comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(I) Compounds
Copper is a chemical element; it has Chemical symbol, symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductility, ductile metal with very high thermal conductivity, thermal and electrical conductivity. A freshly exposed surface of pure copper has a Copper (color), pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material#Metal, building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Copper is one of the few metals that can occur in nature in a directly usable, unalloyed metallic form. This means that copper is a native metal. This led to very early human use in several regions, from . Thousands of years later, it was the first metal to be Smelting, smelted from sulfide ores, ; the first metal to be cast into a shape in a mold, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocupration
A hydrocupration is a chemical reaction whereby a ligated copper hydride species (Cu(I)H), reacts with a carbon-carbon or carbon-oxygen pi-system; this insertion is typically thought to occur via a four-membered ring transition state, producing a new copper-carbon or copper-oxygen sigma-bond and a stable (generally) carbon-hydrogen sigma-bond. In the latter instance (copper-oxygen), protonation (protodemetalation) is typical – the former (copper-carbon) has broad utility. The generated copper-carbon bond ( organocuprate) has been employed in various nucleophilic additions to polar conjugated and non-conjugated systems and has also been used to forge (by way of reductive elimination or transmetalation) new carbon-heteroatom bonds (Nitrogen, Boron, etc.). History While copper (I) hydride was the earliest known binary metal hydride (1800s), synthetic organic chemist’s interest in the reactivity of copper hydride complexes did not arise until nearly a century later; this interes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation. When their existence is indicated, reactive intermediates can help explain how a chemical reaction takes place. Most chemical reactions take more than one elementary step to complete, and a reactive intermediate is a high-energy, hence unstable, product that exists only in one of the intermediate steps. The series of steps together make a reaction mechanism. A reactive intermediate differs from a reactant or product or a simple reaction intermediate only in that it cannot usually be isolated but is sometimes observable only through fast spectroscopic methods. It is stable in the sense that an elementary reaction forms the reactive intermediate and the elementary rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benjamin F
Benjamin ( ''Bīnyāmīn''; "Son of (the) right") blue letter bible: https://www.blueletterbible.org/lexicon/h3225/kjv/wlc/0-1/ H3225 - yāmîn - Strong's Hebrew Lexicon (kjv) was the younger of the two sons of Jacob and Rachel, and Jacob's twelfth and youngest son overall in Jewish, Christian and Islamic tradition. He was also considered the progenitor of the Israelite Tribe of Benjamin. Unlike Rachel's first son, Joseph, Benjamin was born in Canaan according to biblical narrative. In the Samaritan Pentateuch, Benjamin's name appears as "" (Samaritan Hebrew: , "son of days"). In the Quran, Benjamin is referred to as a righteous young child, who remained with Jacob when the older brothers plotted against Joseph. Later rabbinic traditions name him as one of four ancient Israelites who died without sin, the other three being Chileab, Jesse and Amram. Name The name is first mentioned in letters from King Sîn-kāšid of Uruk (1801–1771 BC), who called himself “King of Amnanu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
19 Piscium
TX Piscium (19 Piscium) is a variable red giant star in the constellation Pisces. It is amongst the reddest naked eye stars, with a significant reddish hue when seen in binoculars. It is approximately 800 light years from Earth. It is close to—and sometimes considered part of—the asterism on the western end of the constellation called the ''circlet of Pisces''. Spectrum TX Piscium is a very red star, 2.6 magnitudes fainter at blue wavelengths than in the middle of the visual range, and another 3.3 magnitudes fainter in the ultraviolet. It has been given a spectral class C7,2, indicating a relatively cool carbon star with only modest C2 band strength. It has alternately been classified as C-N5 C24, suggesting a warmer star with stronger C2 bands. Spectral features have been observed to vary. Variability The apparent magnitude of TX Piscium varies between +4.9 and +5.5 and it is classified as a slow irregular variable. Photometry has shown some periodicity in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hollow-cathode Lamp
A hollow-cathode lamp (HCL) is type of cold cathode lamp used in physics and chemistry as a spectral line source (e.g. for atomic absorption spectrometers) and as a frequency tuner for light sources such as lasers. An HCL takes advantage of the hollow cathode effect, which causes conduction at a lower voltage and with more current than a cold cathode lamp that does not have a hollow cathode. An HCL usually consists of a glass tube containing a cathode, an anode, and a buffer gas (usually a noble gas). A large voltage across the anode and cathode will cause the buffer gas to ionize, creating a plasma. The buffer gas ions will then be accelerated into the cathode, sputtering off atoms from the cathode. Both the buffer gas and the sputtered cathode atoms will in turn be excited by collisions with other atoms/particles in the plasma. As these excited atoms decay to lower states, they will emit photons. These photons will then excite the atoms in the sample, which will release ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Aluminium Hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula or . It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage. Properties, structure, preparation LAH is a colourless solid but commercial samples are usually gray due to contamination. This material can be purified by recrystallization from diethyl ether. Large-scale purifications employ a Soxhlet extractor. Commonly, the impure gray material is used in synthesis, since the impurities are innocuous and can be easily separated from the organic products. The pure powdered material is pyrophoric, but not its large crystals. Some commercial materials contain mineral oil to inhibit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper Iodide
Copper(I) iodide is an inorganic compound with the chemical formula . It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding. Copper(I) iodide is white, but samples often appear tan or, when found in nature as rare mineral marshite, reddish brown, but such color is due to the presence of impurities. It is common for samples of iodide-containing compounds to become discolored due to the facile aerobic oxidation of the iodide anion to molecular iodine. Structure Copper(I) iodide, like most binary (containing only two elements) metal halides, is an inorganic polymer. It has a rich phase diagram, meaning that it exists in several crystalline forms. It adopts a zinc blende structure below 390 °C (γ-CuI), a wurtzite structure between 390 and 440 °C (β-CuI), and a rock salt structure above 440 °C (α-CuI). The ions are tetrahedrally coordinated when in the zinc blende or the wurtzite structure, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofunctionalization
A hydrofunctionalization reaction is the addition of hydrogen and another univalent fragment (X) across a carbon-carbon or carbon-heteroatom multiple bond. Often, the term ''hydrofunctionalization'' without modifier refers specifically to the use of the covalent hydride (H-X) as the source of hydrogen and X for this transformation. If other reagents are used to achieve the net addition of hydrogen and X across a multiple bond, the process may be referred to as a ''formal hydrofunctionalization''. For terminal olefins (or acetylenes), the regioselectivity of the process can be described as Markovnikov (addition of X at the substituted end) or anti-Markovnikov (addition of X at the unsubstituted end). Catalysts are frequently employed to control the chemo-, regio-, and stereoselectivity of hydrofunctionalization reactions. Examples Some of the better known classes of hydrofunctionalization reactions include the following: * Hydroboration * Hydrosilylation * Hydrometalation (in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymethylhydrosiloxane
Polymethylhydrosiloxane (PMHS) is a polymer with the general structure . It is used in organic chemistry as a mild and stable reducing agent easily transferring hydrides to metal centers and a number of other reducible functional groups. A variety of related materials are available under the following CAS registry numbers 9004-73-3, 16066-09-4, 63148-57-2, 178873-19-3. These include the tetramer (), copolymers of dimethylsiloxane and methylhydrosiloxane, and trimethylsilyl terminated materials. This material is prepared by the hydrolysis of monomethyldichlorosilane CAS#: 75-54-7: : The related polymer polydimethylsiloxane (PDMS) is made similarly, but lacking bonds, it exhibits no reducing properties. Dimethyldichlorosilane CAS#: 75-78-5 is then used instead of monomethyldichlorosilane CAS#: 75-54-7. Illustrative of its use, PMHS is used for ''in situ'' conversion of tributyltin oxide to tributyltin hydride Tributyltin hydride is an organotin compound with the formula (C4H9) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |