|
Computational Chemistry
Computational chemistry is a branch of chemistry that uses computer simulations to assist in solving chemical problems. It uses methods of theoretical chemistry incorporated into computer programs to calculate the structures and properties of molecules, groups of molecules, and solids. The importance of this subject stems from the fact that, with the exception of some relatively recent findings related to the hydrogen molecular ion (dihydrogen cation), achieving an accurate quantum mechanical depiction of chemical systems analytically, or in a closed form, is not feasible. The complexity inherent in the many-body problem exacerbates the challenge of providing detailed descriptions of quantum mechanical systems. While computational results normally complement information obtained by chemical experiments, it can occasionally predict unobserved chemical phenomena. Overview Computational chemistry differs from theoretical chemistry, which involves a mathematical description of chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C60 Isosurface
C6, C06, C VI or C-6 may refer to: Vehicles Road * Chevrolet Corvette C6, a 2005 sports car * Cierva C.6, a 1924 Spanish autogyro * Citroën C6, a 2005 executive car * Sauber SHS C6, a 1982 Group C prototype racing car * Ford C6 transmission, a heavy-duty automatic transmission built by Ford Motor Company Air * AEG C.VI, a German World War I reconnaissance aircraft * DFW C.VI, a 1916 German reconnaissance aircraft * LVG C.VI, a 1917 German twin-seat reconnaissance aircraft * C-6 Ute, a military version of the Beechcraft King Air airplane Rail * Bavarian C VI, an 1899 German steam locomotive model * LNER Class C6, a class of British 3-cylinder compound locomotives Water * C-6, United States Army designation of the Sikorsky S-38 amphibious flying boat * HMS C6, HMS ''C6'', a 1906 British Royal Navy submarine * USS Olympia (C-6), USS ''Olympia'' (C-6), a 1892 United States Navy protected cruiser Anatomy and medicine * Complement component 6 (C6 protein) in the complement cascade sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Edgar Bright Wilson
Edgar Bright Wilson Jr. (December 18, 1908 – July 12, 1992) was an American chemist. Wilson was a prominent and accomplished chemist and teacher, recipient of the National Medal of Science in 1975, Guggenheim Fellowships in 1949 and 1970, the Elliott Cresson Medal in 1982, and a number of honorary doctorates. He was a member of both the American Academy of Arts and Sciences, the American Philosophical Society, and the United States National Academy of Sciences. He was also the Theodore William Richards Professor of Chemistry, Emeritus at Harvard University. One of his sons, Kenneth G. Wilson, was awarded the Nobel Prize in physics in 1982. E. B. Wilson was a student and protégé of Nobel laureate Linus Pauling and was a coauthor with Pauling of ''Introduction to Quantum Mechanics'', a graduate level textbook in Quantum Mechanics. Wilson was also the thesis advisor of Nobel laureate Dudley Herschbach. Wilson was elected to the first class of the Harvard Society of Fellows. W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azulene
Azulene is an aromatic organic compound and an isomer of naphthalene. Naphthalene is colourless, whereas azulene is dark blue. The compound is named after its colour, as "azul" is Spanish for blue. Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that feature the azulene skeleton are found in nature as constituents of pigments in mushrooms, guaiac wood oil, and some marine invertebrates. Azulene has a long history, dating back to the 15th century as the azure-blue chromophore obtained by steam distillation of German chamomile. The chromophore was discovered in yarrow and wormwood and named in 1863 by Septimus Piesse. Its structure was first reported by Lavoslav Ružička, followed by its organic synthesis in 1937 by Placidus Plattner. Structure and bonding left, The blue color of the mushroom '' Lactarius indigo'' is due to the azulene derivative (7-isopropenyl-4-methylazulen-1-yl)methyl stearate. Azulene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white Crystal, crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 Parts-per notation, ppm by mass. As an Aromaticity, aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is the main ingredient of traditional mothballs. History In the early 1820s, two separate reports described a white solid with a pungent odor derived from the distillation of coal tar. In 1821, John Kidd (chemist), John Kidd cited these two disclosures and then described many of this substance's properties and the means of its production. He proposed the name ''naphthaline'', as it had been derived from a kind of naphtha (a broad term encompassing any volatile, flammable liquid hydrocarbon mixture, including coal tar). Naphthalene's chemical formula was determined by Michael Faraday in 1826. The structure of two f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Francis Boys
Samuel Francis (Frank) Boys (20 December 1911 – 16 October 1972) was a British theoretical chemist. Education Boys was born in Pudsey, Yorkshire, England. He was educated at the Grammar School in Pudsey and then at Imperial College London, whence he graduated in Chemistry in 1932. He then embarked on postgraduate study at Trinity College, Cambridge, supervised first by Martin Lowry, and then, after Lowry's death in 1936, by John Lennard-Jones. He awarded a PhD in 1937 from Cambridge, for a thesis on "The Quantum Theory of Optical Rotation". Career In 1938, Boys was appointed an Assistant Lecturer in Mathematical Physics at Queen's University Belfast. He spent the whole of the Second World War working on explosives research with the Ministry of Supply at the Royal Arsenal, Woolwich, with Lennard-Jones as his supervisor. After the war, Boys accepted an ICI Fellowship at Imperial College, London. In 1949, he was appointed to a Lectureship in theoretical chemistry at the Un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EDSAC
The Electronic Delay Storage Automatic Calculator (EDSAC) was an early British computer. Inspired by John von Neumann's seminal ''First Draft of a Report on the EDVAC'', the machine was constructed by Maurice Wilkes and his team at the University of Cambridge Mathematical Laboratory in England to provide a service to the university. EDSAC was the second electronic digital stored-program computer, after the Manchester Mark 1, to go into regular service. Later the project was supported by J. Lyons and Co., J. Lyons & Co. Ltd., intending to develop a commercially applied computer and resulting in Lyons' development of the LEO (computer), LEO I, based on the EDSAC design. Work on EDSAC started during 1947, and it ran its first programs on 6 May 1949, when it calculated a table of square numbers and a list of prime numbers. EDSAC was finally shut down on 11 July 1958, having been superseded by EDSAC 2, which remained in use until 1965. Project and plan The concept ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Configuration Interaction
Configuration interaction (CI) is a post-Hartree–Fock linear variational method for solving the nonrelativistic Schrödinger equation within the Born–Oppenheimer approximation for a quantum chemical multi-electron system. Mathematically, ''configuration'' simply describes the linear combination of Slater determinants used for the wave function. In terms of a specification of orbital occupation (for instance, (1s)2(2s)2(2p)1...), ''interaction'' means the mixing (interaction) of different electronic configurations (states). Due to the long CPU time and large memory required for CI calculations, the method is limited to relatively small systems. In contrast to the Hartree–Fock method, in order to account for electron correlation, CI uses a variational wave function that is a linear combination of configuration state functions (CSFs) built from spin orbitals (denoted by the superscript ''SO''), : \Psi = \sum_ c_ \Phi_^ = c_0\Phi_0^ + c_1\Phi_1^ + where � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gaussian Orbital
In computational chemistry and molecular physics, Gaussian orbitals (also known as Gaussian type orbitals, GTOs or Gaussians) are functions used as atomic orbitals in the LCAO method for the representation of electron orbitals in molecules and numerous properties that depend on these. Rationale The use of Gaussian orbitals in electronic structure theory (instead of the more physical Slater-type orbitals) was first proposed by Boys in 1950. The principal reason for the use of Gaussian basis functions in molecular quantum chemical calculations is the 'Gaussian Product Theorem', which guarantees that the product of two GTOs centered on two different atoms is a finite sum of Gaussians centered on a point along the axis connecting them. In this manner, four-center integrals can be reduced to finite sums of two-center integrals, and in a next step to finite sums of one-center integrals. The speedup by 4-5 orders of magnitude compared to Slater orbitals outweighs the extra cost ent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Slater Orbital
Slater-type orbitals (STOs) or Slater-type functions (STFs) are functions used as atomic orbitals in the linear combination of atomic orbitals molecular orbital method. They are named after the physicist John C. Slater, who introduced them in 1930. They possess exponential decay at long range and Kato's cusp condition at short range (when combined as hydrogen-like atom functions, i.e. the analytical solutions of the stationary Schrödinger equation for one electron atoms). Unlike the hydrogen-like ("hydrogenic") Schrödinger orbitals, STOs have no radial nodes (neither do Gaussian-type orbitals). Definition STOs have the following radial part: : R(r) = N r^ e^\, where * is a natural number that plays the role of principal quantum number, = 1,2,..., * is a normalizing constant, * is the distance of the electron from the atomic nucleus, and * \zeta is a constant related to the effective charge of the nucleus, the nuclear charge being partly shielded by electrons. Historically ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Basis Set (chemistry)
In theoretical chemistry, theoretical and computational chemistry, a basis set is a set of Function (mathematics), functions (called basis functions) that is used to represent the Wave function, electronic wave function in the Hartree–Fock method or Density functional theory, density-functional theory in order to turn the partial differential equations of the model into algebraic equations suitable for efficient implementation on a computer. The use of basis sets is equivalent to the use of an approximate resolution of the identity: the Atomic orbital, orbitals , \psi_i\rangle are expanded within the basis set as a linear combination of the basis functions , \psi_i\rangle \approx \sum_\mu c_ , \mu\rangle, where the expansion coefficients c_ are given by c_ = \sum_\nu \langle \mu, \nu \rangle^ \langle \nu , \psi_i \rangle. The basis set can either be composed of atomic orbitals (yielding the linear combination of atomic orbitals approach), which is the usual choice within the qua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
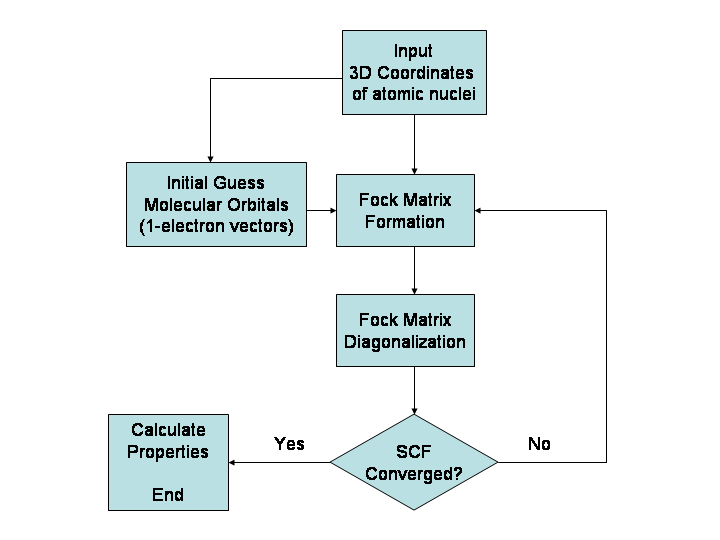
Hartree–Fock Method
In computational physics and chemistry, the Hartree–Fock (HF) method is a method of approximation for the determination of the wave function and the energy of a quantum many-body system in a stationary state. The method is named after Douglas Hartree and Vladimir Fock. The Hartree–Fock method often assumes that the exact ''N''-body wave function of the system can be approximated by a single Slater determinant (in the case where the particles are fermions) or by a single permanent (in the case of bosons) of ''N'' spin-orbitals. By invoking the variational method, one can derive a set of ''N''-coupled equations for the ''N'' spin orbitals. A solution of these equations yields the Hartree–Fock wave function and energy of the system. Hartree–Fock approximation is an instance of mean-field theory, where neglecting higher-order fluctuations in order parameter allows interaction terms to be replaced with quadratic terms, obtaining exactly solvable Hamiltonians. Especially ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element. Atoms are extremely small, typically around 100 picometers across. A human hair is about a million carbon atoms wide. Atoms are smaller than the shortest wavelength of visible light, which means humans cannot see atoms with conventional microscopes. They are so small that accurately predicting their behavior using classical physics is not possible due to quantum mechanics, quantum effects. More than 99.94% of an atom's mass is in the nucleus. Protons hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |